Publications


186. Pootheri, N.; Lee, S.* “Palladium-Catalyzed One-Pot Synthesis of N‑Formylaniline Derivatives Using Oxalic Acid as a Dual Carbon Monoxide and Hydrogen Donor” Org. Lett. 2024, 26(43), 9407-9412 [Suppl. Cover]
Synfacts2025, 21(01), 32. DOI: 10.1055/a-2467-5693
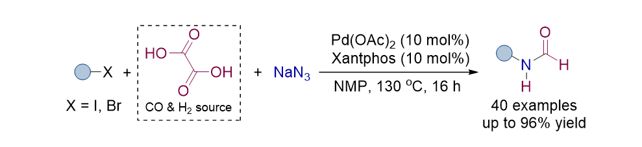

185. An, Y.; Oh, J.;
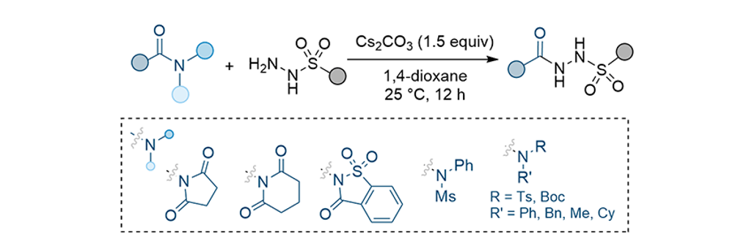
Lee, S.* “Synthesis of N-Acyl-N′-Sulfonyl Hydrazides from Sulfonyl Hydrazides and
Activated Amides” Synthesis, 2024, 56(22), 3468-3474.

184. Pootheri, N.; Lee,
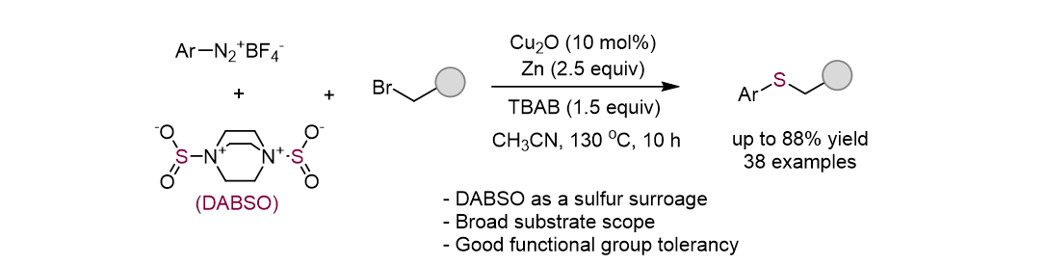
S. “Synthesis of Aryl Alkyl Thioethers via a Copper-Catalyzed Three-Component
Reaction with DABSO, Aryldiazonium Salts, and Alkyl Bromides” J. Org. Chem.2024, 89(19), 14549-14557. [Suppl. Cover]

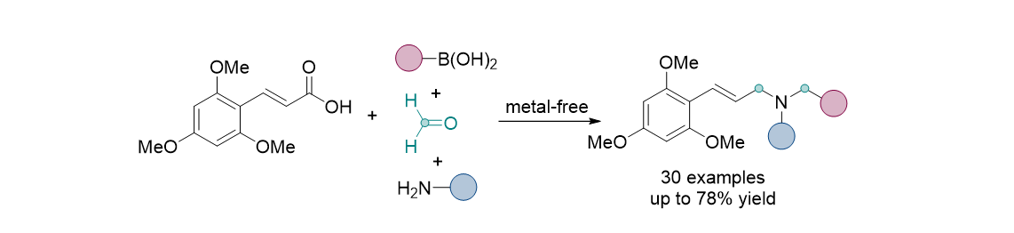
183. Lee, H.; Song, K. H.; Lee, S.* “Integrated Approach for Allyl Amine Synthesis Combining the Decarboxylative Coupling of Arylacrylic Acids with the Petasis Reaction.” J. Org. Chem. 2024, 89(19), 14527-14536 [Suppl. Cover]

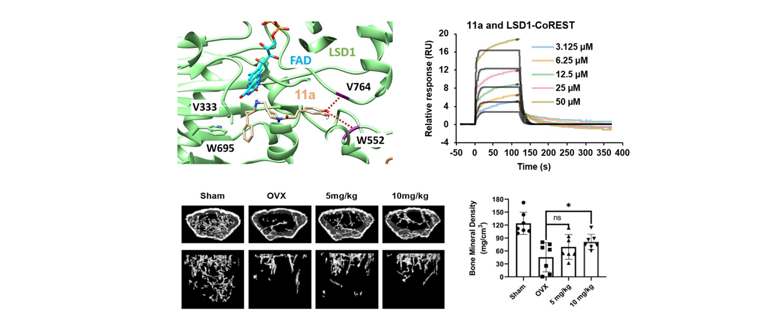
182. Chen, Z.; Choi, E.
R.; Encarnacion, A. M.; Yoo, H.; Ding, M.; Park, Y.-H.; Choi, S. M.; An, Y. J.;
Hong, E.; Choi, H.-H.; Kim, S. K.; Nam, Y. E.; Kim, G.-J.; Park, S.-w.; Kim,
J.-S.; Kim, E.: Lee, S.; Cho, J. H.*; Lee, T.* “Discovery of TCP-(MP)-caffeic
acid analogs as a new class of agents for treatment of osteoclastic bone loss”
Bioorg. Chem. 2024, 150, 107603

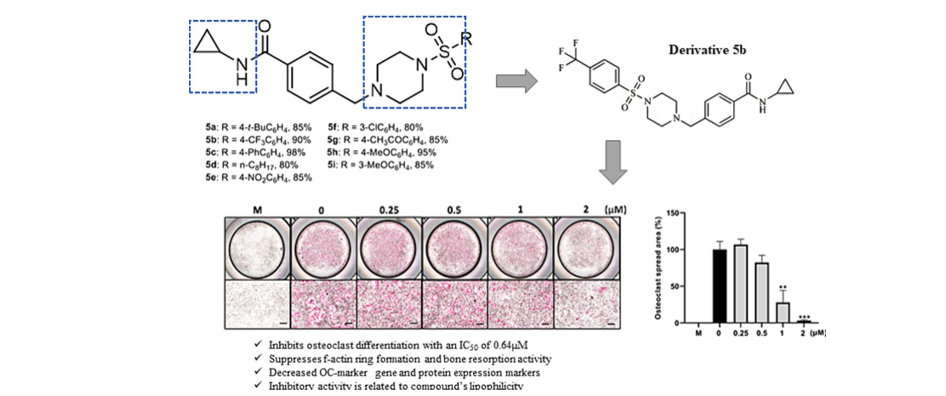
181. Encarnacion, A.
M.; Pootheri, N.; Yao, H.; Chen, Z.; Lee, S.; Kim, S.; Lee, T.-H. “Novel
Inhibitor
N-cyclopropyl-4-((4-((4-(trifluoromethyl)phenyl) sulfonyl)piperazin-1-yl)methyl)benzamide
Attenuates RANKL-Mediated Osteoclast Differentiation In Vitro”

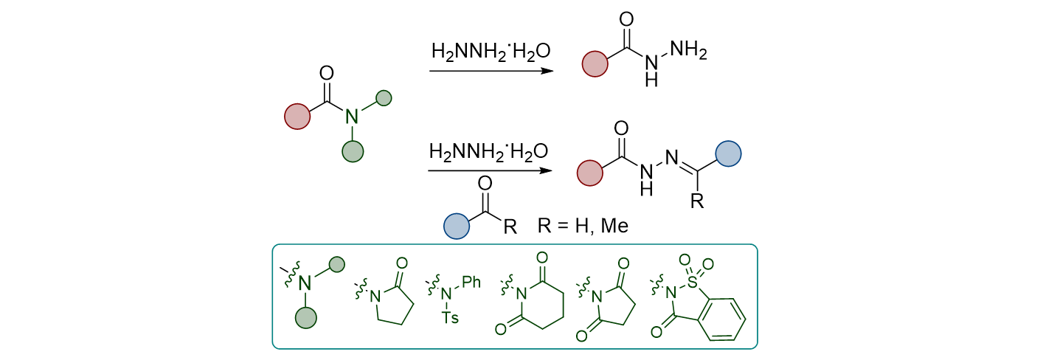
180. Kim, M.; Lee, S. “Synthesis
of Acyl Hydrazides and Hydrazones from Activated Amides” Synthesis, 2024, 56(14),
2263-2269.

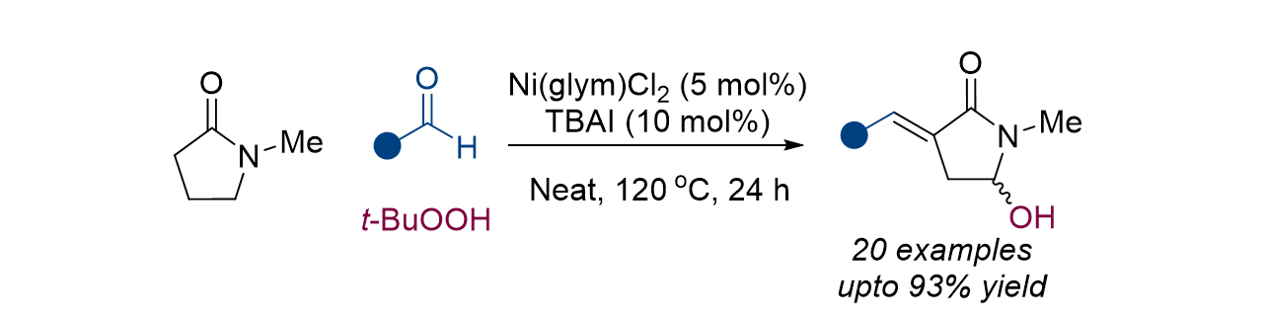
179. Rajan, R. P. S.; Mok, J.; Lee, J.; Lee, S.* “One-Step Nickel-Catalyzed Synthesis of Alpha-Arylidene and Gamma Hydroxy-Substituted N-Methyl-2-Pyrrolidones” Asian, J. Org. Chem. 2024, 13, e202400070. [Front Cover]

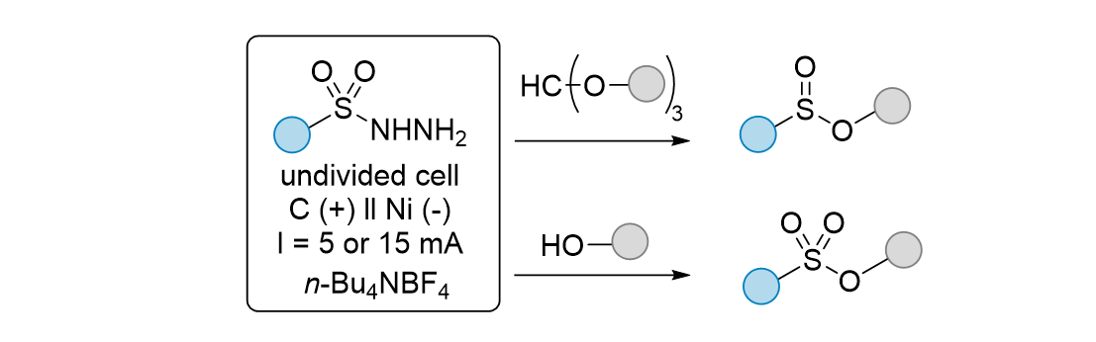
178. Kim, S.; Lee, S.* “Electrochemical synthesis of sulfinic and sulfonic esters from sulfonyl hydrazides” Org. Biomol. Chem. 2024, 22, 4436-4444. [Front Cover]

177. Chen, Z.;
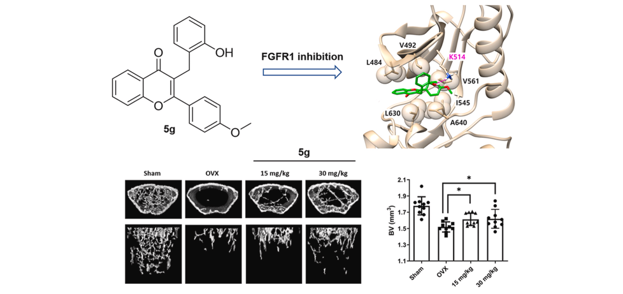
Encarnacion, A. M.; Rajan, R. P. S.; Yao, H.; Lee, S.; Kim, E.; Lee, T.-H. “Discovery
of a novel homoisoflavonoid derivative 5g for anti-osteoclastic bone loss via
targeting FGFR1” Eur. J. Med. Chem. 2024, 270, 116335.

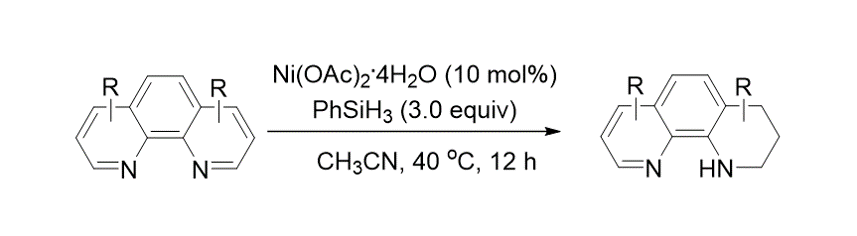
176. An, Y.; Lee, S.; Oh, J. “Nickel-Catalyzed Reduction of Aromatic Ring of Phenanthrolines” J. Korean Chem. Soc. 2024, 68(1), 12-14

175. Rajamanickam, K. R.; Lee, S.* “Ring Opening of N-Acyl Lactams Using Nickel-Catalyzed Transamidation” J. Org. Chem. 2024, 89(2), 1336-1344.[Cover image]

174. Go, G. Y.; Rajan,
R. P. S.; Lee, S.*; Choi, H. C.* “Molybdenum trioxide-decorated carbon nanotubes:
an efficient and reusable catalyst for cross-dehydrogenative coupling-type
aza-Henry reaction in aqueous media” Mol. Cat. 2024, 552, 113681



173. Park, M. S.;
Rajamanickam, K. R.; Song, K. H.; Lee, S.* “1,3-Diketone-Mediated Synthesis of
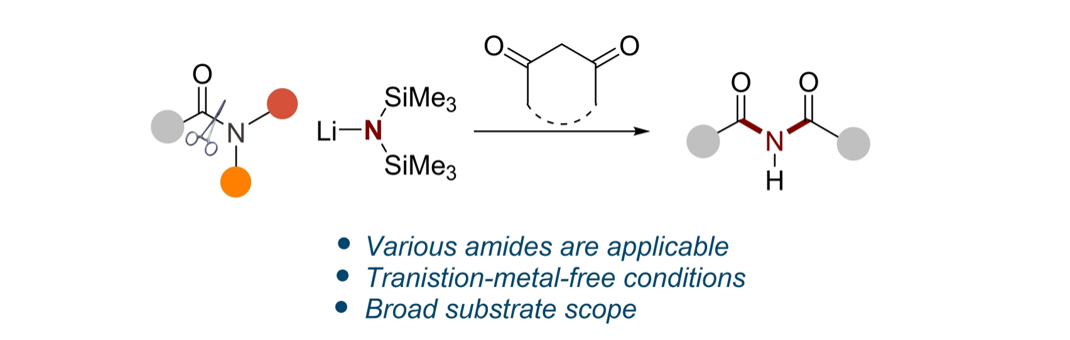
Symmetrical Aryl Imides from Activated Amides” Asian J. Org. Chem. 2023, 12, e2023004

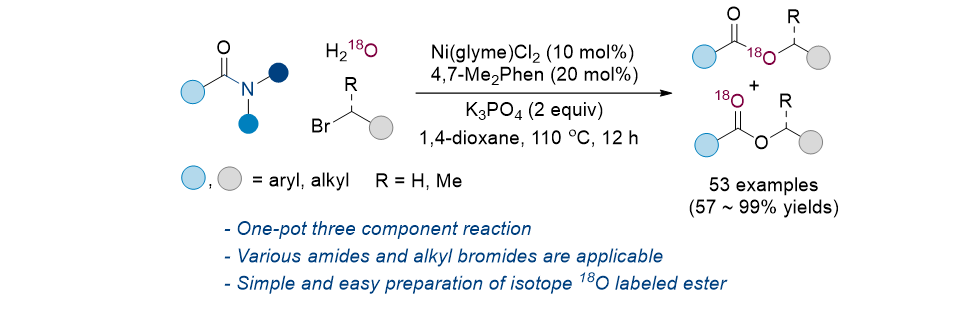
172. Pootheri, N.; Lee,
S.* “Nickel-Catalyzed Isotopic Labeling: Synthesis of Oxygen-18-Labeled Esters
from Amides” Adv. Synth. Catal. 2023, 365(22), 3950-3957.

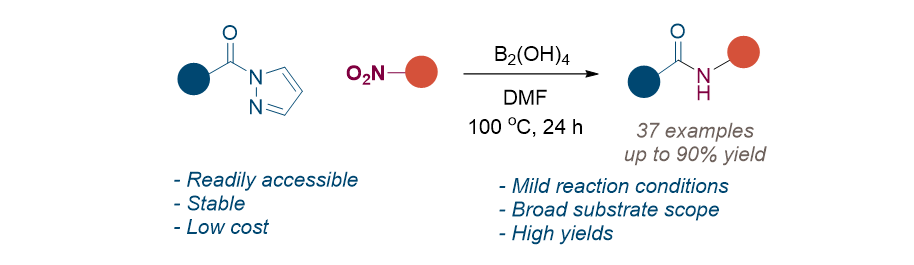
171. Moon, H.; Lee, S.* “Reductive cross-coupling of N-acyl pyrazole and nitroarene using
tetrahydroxydiboron: synthesis of secondary amides” Org. Biomol. Chem. 2023, 21(41),
8329 - 8334.

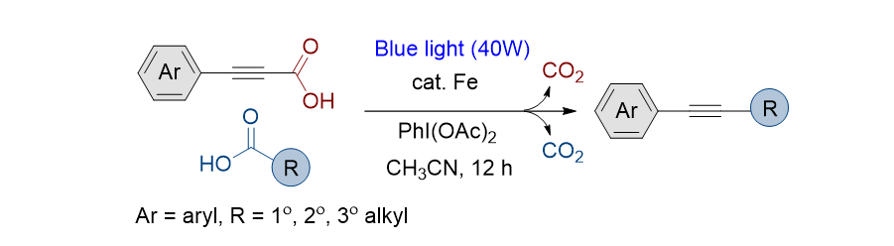
170. Kang, H.; An, S.; Lee, S.* “Iron-Photocatalyzed Double Decarboxylative
Coupling Reactions of Alkynoic Acids and Alkyl Carboxylic Acids: Access to
Alkylated Alkynes” Org. Chem. Front. 2023, 10(20), 5151-5157

169. Chen, Z.; Joseph,
D.; Ding, M.; Bhujbal, S. P.; Rajan, R. P. S.; Kim, E.; Park, S.-w.; Lee, S.*;
Lee, T.-H.* Synthesis and evaluation of 2-NMPA derivatives as potential agents
for prevention of
osteoporosis in vitro and in vivo. Eur. J. Med. Chem. 2023, 260, 115767.

168. Park, H.; Lee, S.*“Palladium and Copper-Catalyzed Friedel–Crafts Acylation with Activated Amides” Adv.
Synth. Catal. 2023, 365(18), 3165-3171 (Inside Cover)


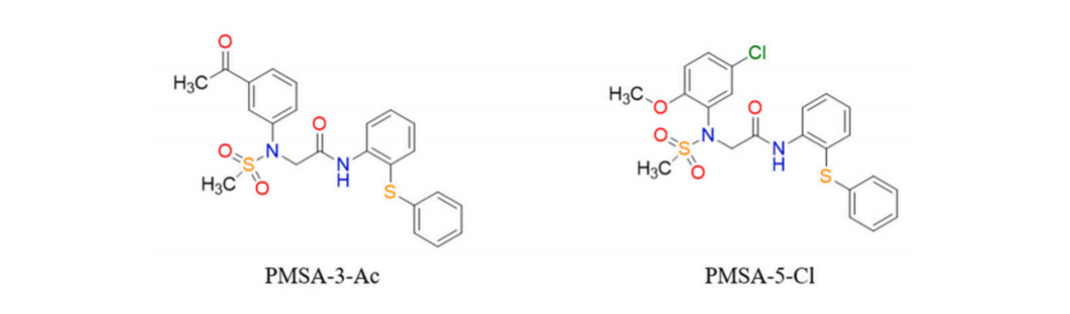
166. Chen, Z.; Rajamanickam, K. R.; Ding, M.; Kim, S. K.; Park, S.-w.; Kim, E.; Lee, S.*; Lee, T.-H.* "Discovery of PMSA Derivative 11 as a Novel Lead Compound for Therapeutic Treatment of Osteoporosis In Vitro and In Vivo" J. Med. Chem. 2023, 66(10), 6766-6781. (Suppl. cover)

165. Bashir, I. A.; Lee, S. "Base-Mediated Synthesis of Anhydrides from Activated Amides" J. Org. Chem. 2023, 88(9), 6159-6167. (Suppl. cover)



164. Idris, M. A.; Lee, S.* “Highly Reactive Palladium-Catalyzed and Acetonitrile-Mediated Three-Component Reactions for Arylsulfone Synthesis” Org. Lett. 2022, 24, 8520-8525 [Cover image]
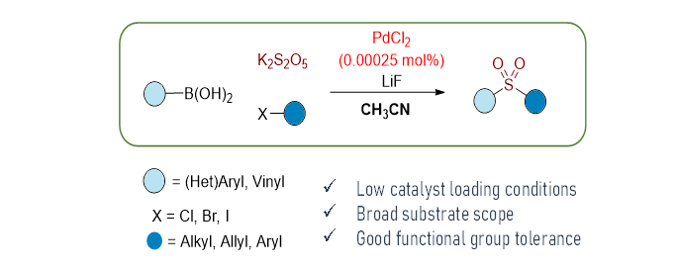

163. Lee, J.; An, S.; Jang, M.; Jung, H. M.*; Lee, S.* "Recyclable and dual active catalyst of copper nanocluster-bound graphitic carbon nitride for the photo-induced synthesis of arylsulfones" Mol. Catal. 2022, 533, 112787.
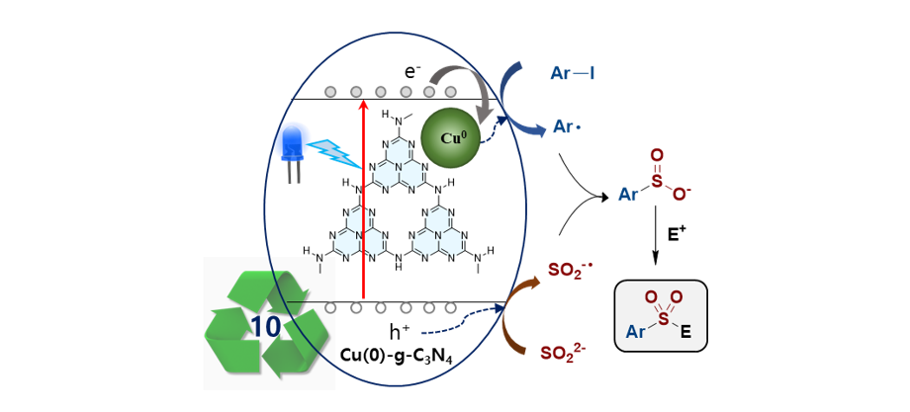

162. Kim, J.; Park, M.
S.; Lee, S.*; Song, K. H.* “Flow reaction system for the synthesis of
benzoylacetonitrile via the reaction of amides and acetonitrile” Tetrahedron
Lett. 2022, 111, 154201.
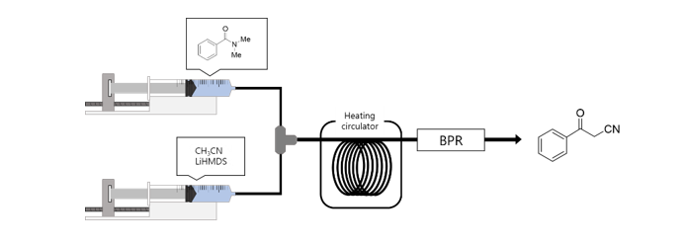

161. Kim, J. Y.; Jo, Y.; Kim, J. D.; Choi, M. Y.; Lee, S.*; Choi, H. C.* “Unveiling the effect of gas treatment on the electronic structure of carbon nanotube-supported Pd catalysts for electroreduction of H2O2 and Heck reaction” Chemosphere 2022, 307, 135838.

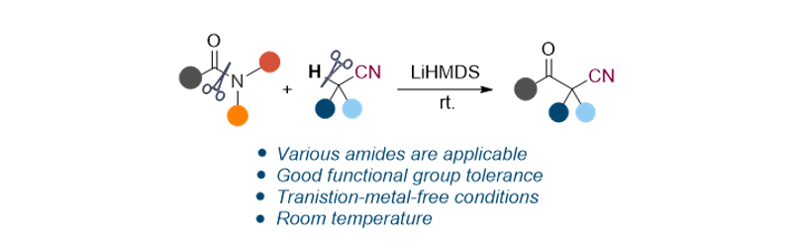
160. Park, M. S.; Lee, S.*“Transition-metal-catalyst-free reaction of amides and acetonitriles: Synthesis of ß-ketonitriles” Org. Chem. Front. 2022, 9(19), 5178-5184.

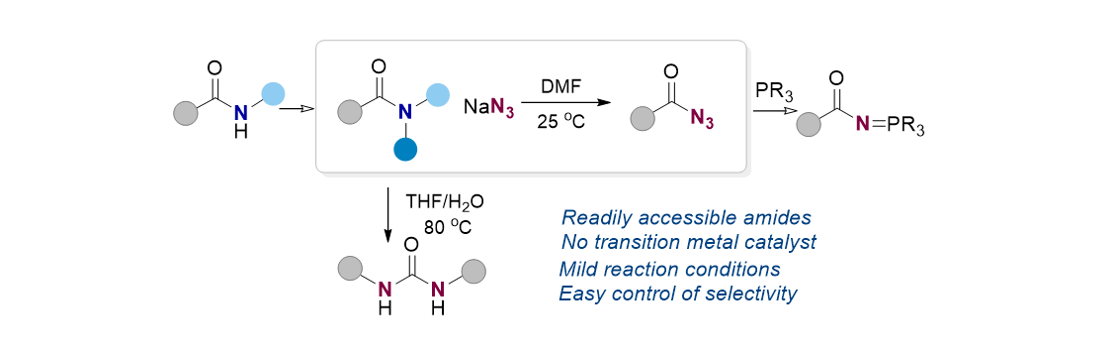
159. Joseph, D.; Lee, S.* “Reaction of Amide and Sodium Azide for the Synthesis of Acyl Azide, Urea, and Iminophosphorane” Org. Lett. 2022, 24(33), 6186-6191. [Suppl. cover image]

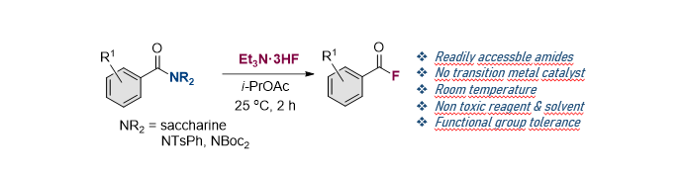
158. Idris, M. A.;
Song, K. H.; Lee, S.* “Synthesis of (Hetero)Aroyl Fluorides via a Mild Amides
C-N Bond Cleavage” Adv. Synth.
Catal. 2022, 364(14), 2449-2453

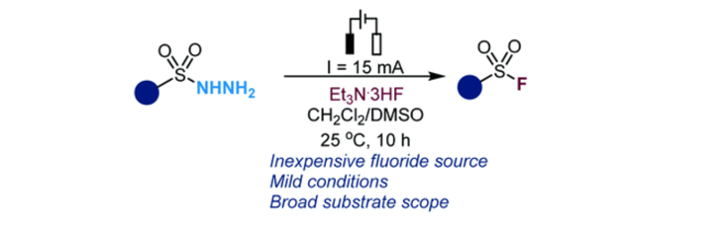
157. Park, J. K.; Oh, J.; Lee, S.* “Electrochemical Synthesis of Sulfonyl Fluorides from Sulfonyl Hydrazides” Org. Chem. Front. 2022, 9(13), 3407-3413. [Front Cover]

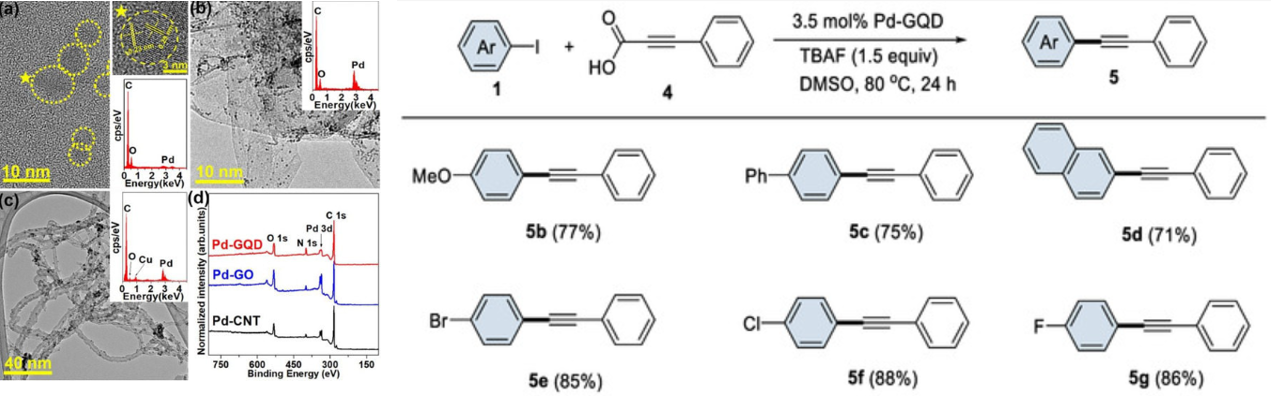
156. Kim,
J. S.; Lim, J.; Kim, J. D.; Choi, M. Y.; Lee, S.*; Choi, H. C.* “Preparation,
characterization, and catalytic properties of Pd-graphene quantum dot catalysts” Catalysts, 2022, 12(6),
619

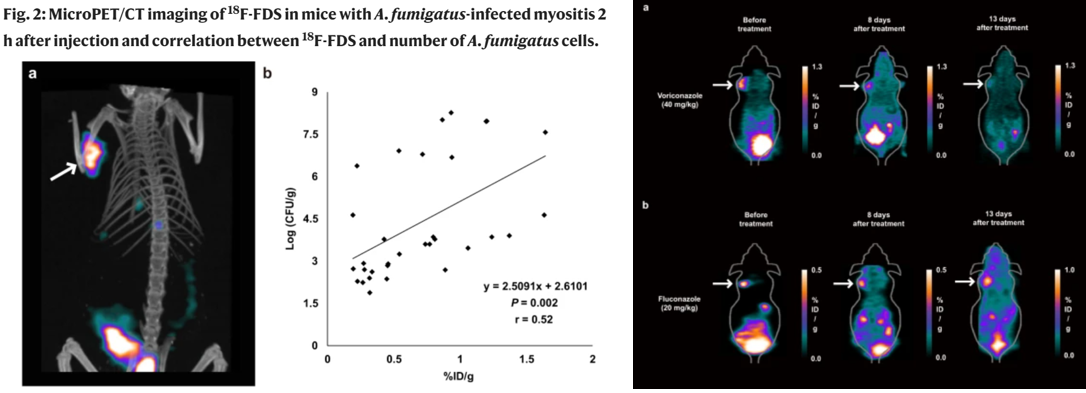
155. Kim, D.-Y.; Pyo,
A.; Ji, S.; You, S.-H.; Kim. S. E.; Lim, D.; Kim, H.; Lee, K.-H.; Oh, S.-J.:
Jung, Y.-r.; Kim U. J.; Jeon, S.; Kwon, S. Y.; Kang, S.-R.; Lee, H. B.; Hyun,
H.; Kim, S.-Y.; Moon, K.-S.; Lee, S.; Kang, S. J.; Min, J.-J. “In vivo imaging
of invasive aspergillosis with 18F-fluorodeoxysorbitol positron emission tomography”
Nat. Commun. 2022, 13, 1926.

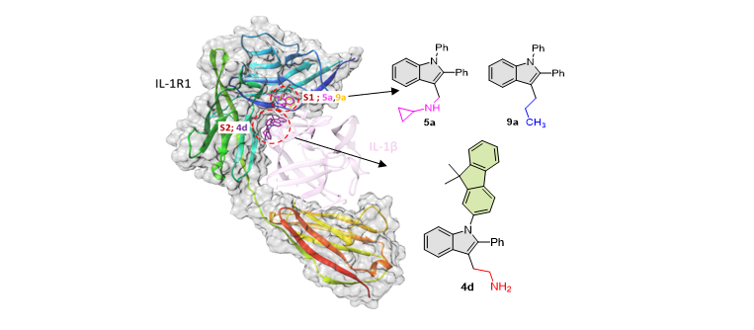
154. Lim, J.; Seon, J.;
Jung, S.; Lee, T.-H.; Kim, E.; Lee, S.* “Synthesis of DPIE
[2-(1,2-Diphenyl-1H-indol-3-yl)ethanamine] Derivatives and Their Regulatory
Effects on Pro-Inflammatory Cytokine Production in IL-1β-Stimulated Primary
Human Oral Cells” Molecules, 2022, 27(3), 899-913



153 . Joseph, D.; Oh, MS; Jayaraman, A.; Lee, S.* “ Amides Activation: Transition Metal-Free Coupling Between CN Activated Amides and Enolizable Amides” Bull. Korean Chem. Soc . 2021 , 42(10) , 1293-1295 [Cover Image]

152. Lim, J.: Lee, S.* “Metal-Free
Doubly Decarboxylative Three-Component Reaction:Synthesis of Propargyl Amines”
Asian J. Org. Chem. 2021, 10(10), 2530-2533
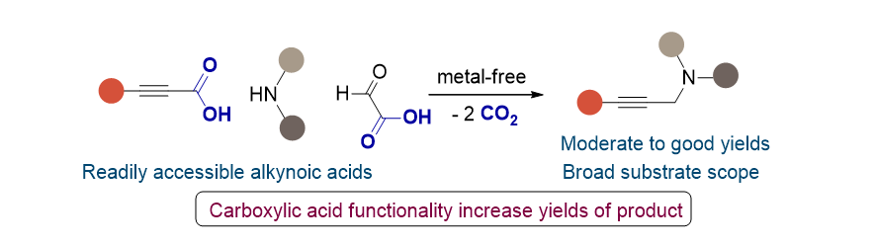
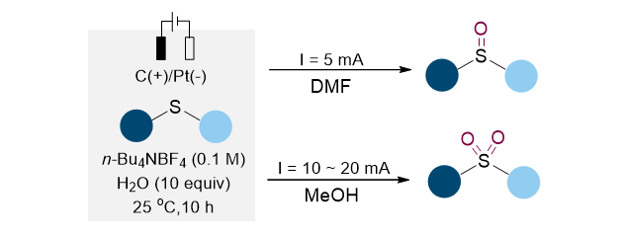
151. Park, J. K.; Lee,
S.* “Sulfoxide and Sulfone Synthesis via Electrochemical Oxidation of Sulfides”J. Org. Chem. 2021, 86(19), 13790-13799.

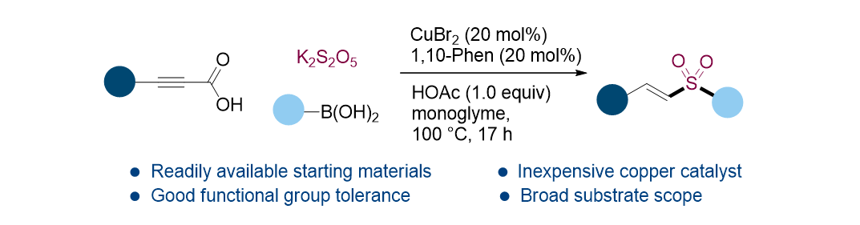
150. An, S.; Song, K.
H.; Lee, S.* “Vinyl sulfone synthesis via copper-catalyzed three-component
decarboxylative addition” Org. Biomol. Chem. 2021, 19(36) 7827-7831.

149. Joseph, D.; Park,
M. S.; Lee, S.* “Metal-free transamidation of benzoylpyrrolidin-2-one and amines
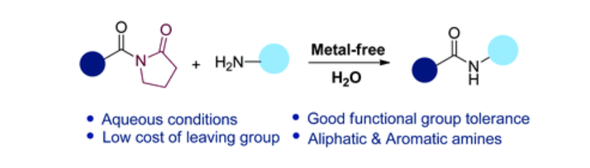
under aqueous conditions” Org. Biomol. Chem. 2021, 19(28) 6227-6232.

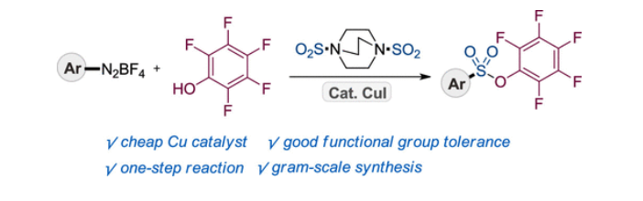
148. Idris,
M. A.: Lee, S.* “One-Pot Synthesis of Pentafluorophenyl Sulfonic Esters via
CopperCatalyzed Reaction of Aryl Diazonium Salts, DABSO, and Pentafluorophenol”
Org. Lett. 2021, 23(12) 4516-4520.[Cover Image]

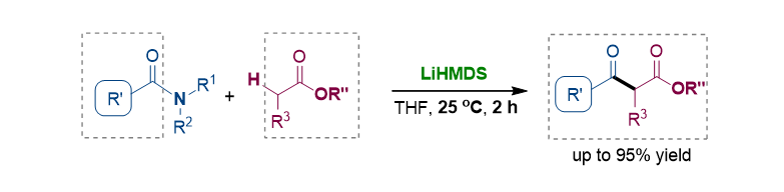
147. Chen, J.; Joseph, D.; Xia, Y.; Lee, S.* “Amide/Ester Cross-Coupling via C–N/C–H Bond Cleavage: Synthesis of β-Ketoesters” J. Org. Chem. 2021, 86(8), 5943-5953. [Cover Image]

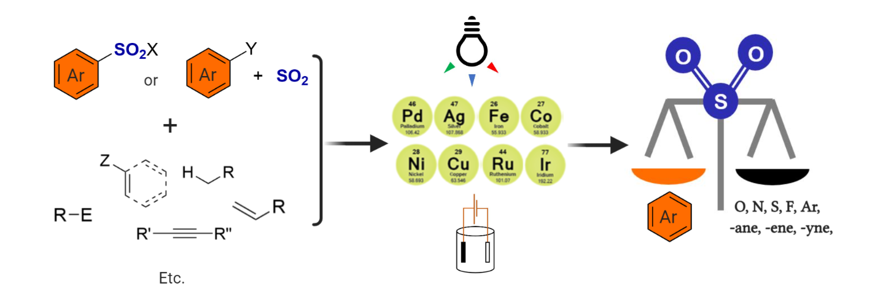
146. Joseph, D.; Idris, M. A.; Chen, J.; Lee, S.* “Recent Advances in the Catalytic Synthesis of Arylsulfonyl Compounds” ACS Catal. 2021, 11,(7) 4169-4204.[Cover Image]

145. Seo, E.; Oh, J.; Lee, S.* “Palladium‐Catalyzed Decarboxylative Homodimerization of Propiolic Acids: Synthesis of 1,3‐Enynes” Bull. Korean Chem. Soc. 2021, 42(3), 514-516 [Invited for Special Issue]

144. Chen, Z.; Ding,
M.; Cho, E.; Seong, J.; Lee, S.; Lee, T. “2-NPPA Mitigates Osteoclastogenesis via
Reducing TRAF6-Mediated c-fos Expression” Front. Pharmacol. 2021, 11, 599081.
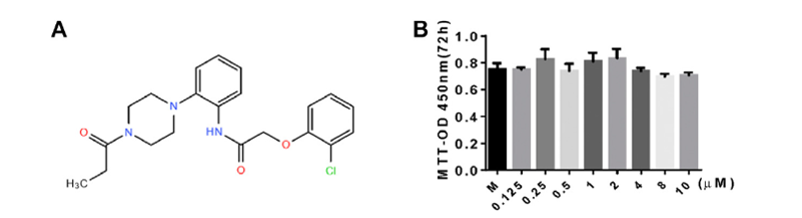

143. Chen, Z.; Cho, E.;
Ding, M.; Seong, J.; Che, X.; Lee, S.; Park, B.-J.; Choi, J.-Y.; Lee, T.-H. “N-[2-(4-benzoyl-1-piperazinyl)phenyl]-2-(4-chlorophenoxy)
acetamide is a novel inhibitor of resorptive bone loss in mice” J. Cell Mol.
Med. 2021, 25, 1425-1438.

142. Cho, E.; Chen, Z.;
Ding, M.; Seong, J.; Lee, S.; Min, S. H.; Choi, D. K.; Lee, T. “PMSA prevents
osteoclastogenesis and estrogen-dependent bone loss in mice” Bone 2021, 142,
115707



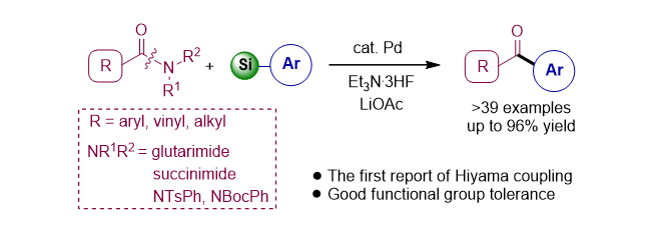
141. Idris, M. A.; Lee, S.* “Palladium-Catalyzed Amide N-C Hiyama Cross-Coupling: Synthesis of Ketones” Org. Lett. 2020, 22(23), 9190-9195. [Cover Art]

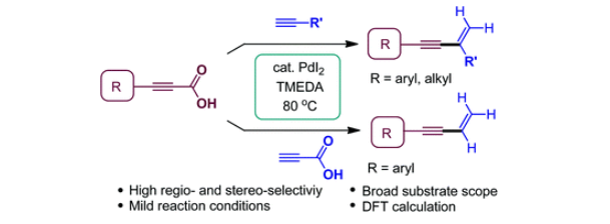
140. Jeon, H.; Ko,
S.-B.; Lee, S.* “Palladium-catalyzed decarboxylative gem-selective addition of
alkynoic acids to terminal alkynes” Org. Chem. Front. 2020, 7(23) 3918-3925

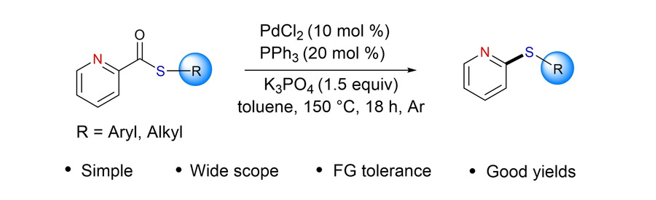
139. Wang, S.-F. Li,
C-E.: Liu, Y.-C.; Reddy, D. M.; Basha, R. S.; Park, J. K.; Lee, S.*; Lee, C.-F.* “Palladium-Catalyzed Decarbonylative Thioetherification of 2- Pyridyl
Thioesters” Asian J. Org. Chem. 2020, 9(11), 1826-1833

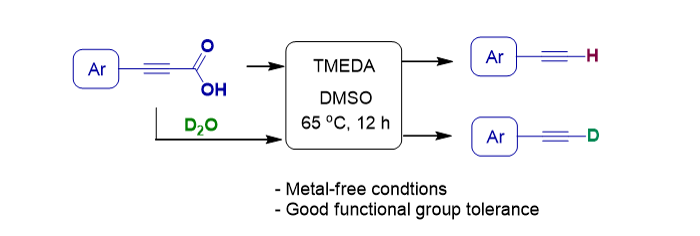
138. Seo, E.; Oh, J.; Lee, S.* “Metal-Free Decarboxylation of Alkynoic Acids for the Synthesis of Terminal Alkynes” Asian J. Org. Chem. 2020, 9(11), 1774-1777 [Cover Picture]

137. Ryu, B.; Oh,
J.; Lee, S.* “Sequential One-Pot Coupling Reactions of Diiodobenzenes, Propiolic
Acid, and Aryl Halides for the Synthesis of Diarylalkynyl Arenes” Asian J. Org.
Chem. 2020, 9(11), 1754-1759
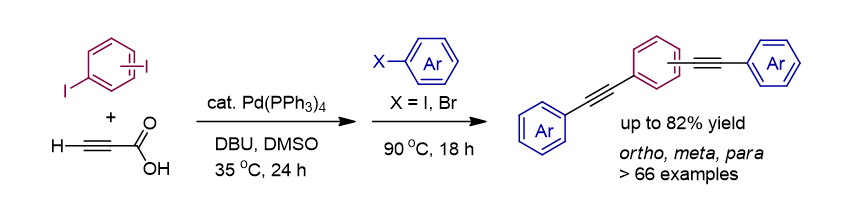

136. Kim, Y.; Song, K.
H.; Lee, S.* “Synthesis of S-Aryl Thioesters via Palladium-Catalyzed Thiocarbonylation
of Aryl Iodides and Aryl Sulfonyl Hydrazides” Org. Chem. Front. 2020, 7(19),
2938-2943.
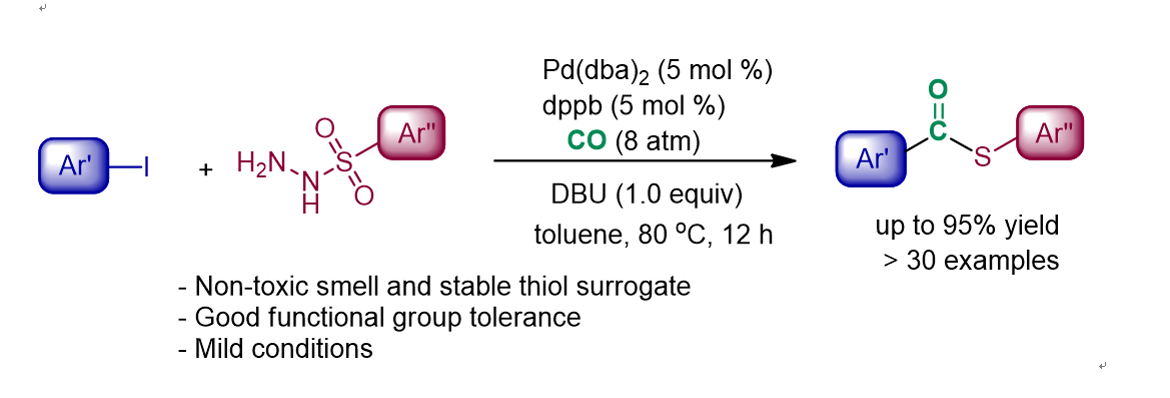

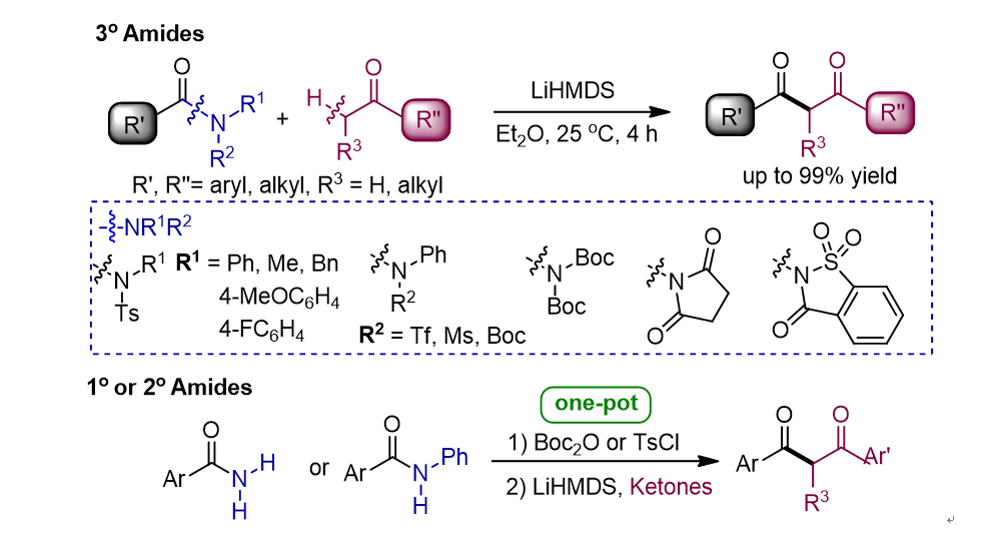

134. Idris, M. A.; Lee,
S.* “Transamidation via C-N Bond Cleavage of Amides and Tertiary Amines” Org.
Chem. Front. 2020, 7(18), 2737-2743.
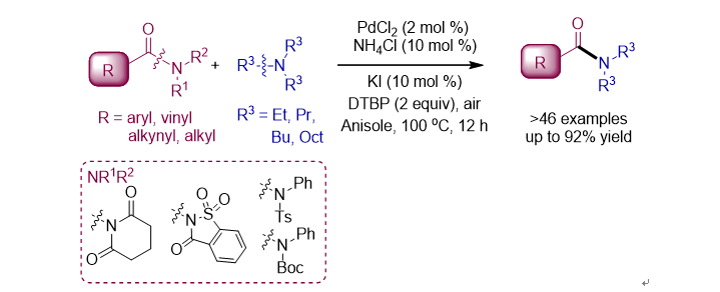

133. Yang, D.; Shin,
T.; Kim, H.; Lee, S.* “Nickel/briphos-catalyzed transamidation of unactivated
tertiary amides” Org. Biomol. Chem. 2020, 18(31), 6053-6057
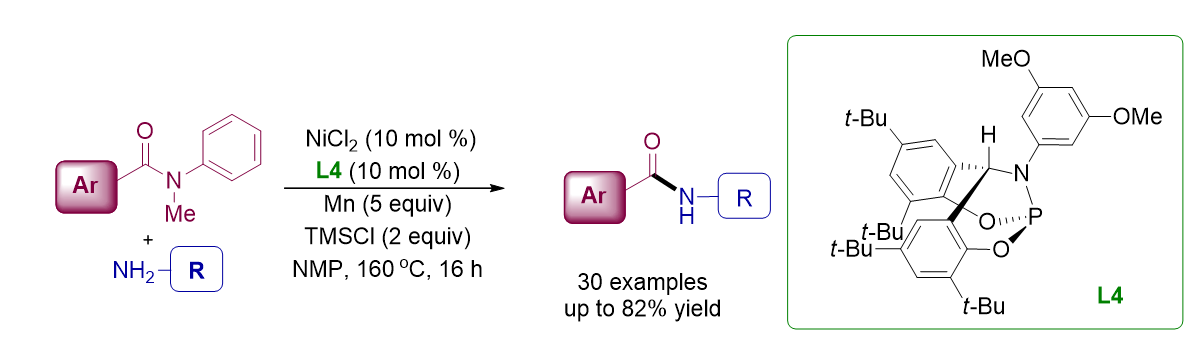

132. Idris, M. A.; Lee, S.* “Recent Advances in
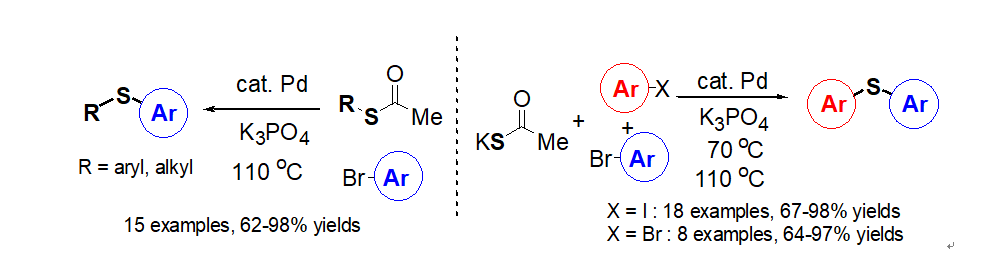
Decarboxylative Reactions of Alkynoic Acids” Synthesis, 2020, 52 (16),
2277-2298. [Invited Reivew]
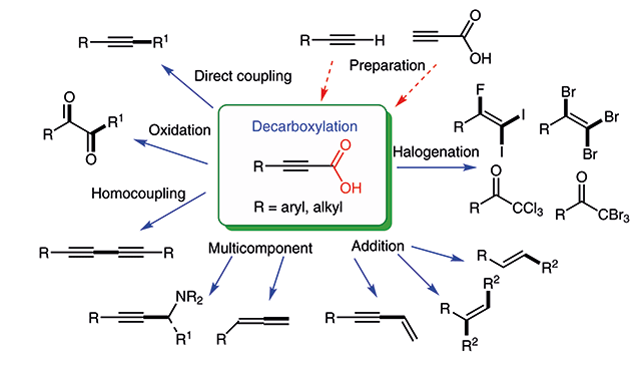

131. Chen, J.; Xia, Y.;
Lee, S.* “Transamidation for the Synthesis of Primary Amides at Room Temperature”
Org. Lett. 2020, 22(9), 3504-3508.
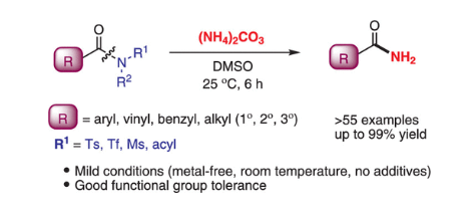

130. Chen, J.; Xu, M.;
Yu, S.; Xia, Y.; Lee, S.* “Nickel-Catalyzed
Claisen Condensation Reaction between Two Different Amides” Org. Lett. 2020, 22
(6), 2287-2292
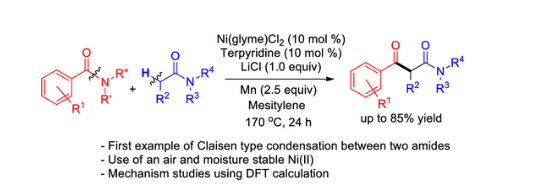

129. Lee, J.; Nam, K. C.; Lee, S.* “Synthesis of aryl allyl alkynes via reaction with allyl amine and aryl alkynoic acids through decarboxylation” Synth. Commun. 2020, 50(7), 1008-1015.
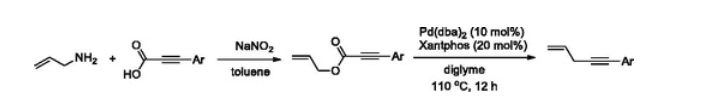



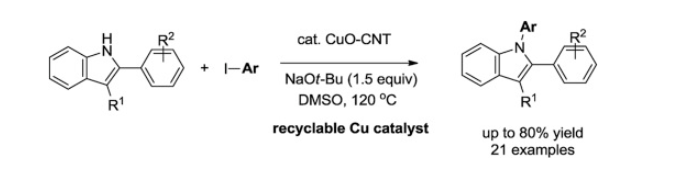
128. Lim, J.; Kim, J.
D.; Choi, H. C.; Lee, S.* “CNT-CuO catalyzed C–N bond formation for N-arylation
of 2-phenylindoles” J. Organomet. Chem. 2019, 902, 120970-120974

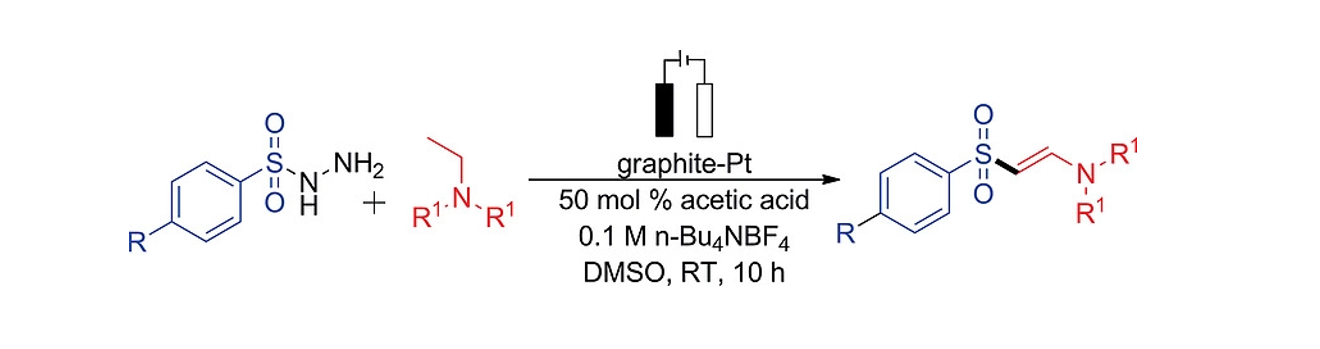
127. Kim, H.-S.; Lee, S.* “Electrochemical Coupling of
Arylsulfonyl Hydrazides and Tertiary Amines for the Synthesis of β-Amidovinyl
Sulfones” Eur. J. Org. Chem. 2019, (41) 6951-6955.

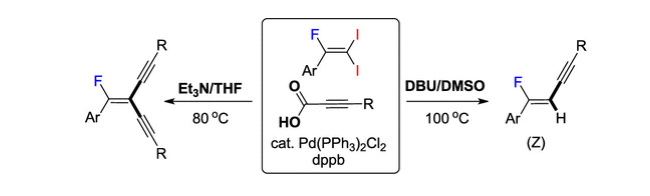
126. Jayaraman, A.; Lee, S.* “Selective Mono- and
Dialkynylation of 1-Fluoro-2,2-diiodovinylarenes Using Pd-Catalyzed
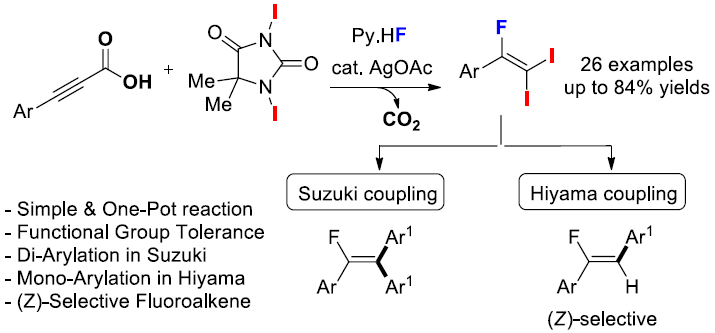
Decarboxylative Coupling Reactions” Org. Lett. 2019, 21 (19), 7923-7927

125. Yu, S.; Song, K.
H.; Lee, S.* “Metal-Free
Transamidation of Primary Amides using Trimethylsilyl Chloride” Asian J. Org.
Chem. 2019, 8(9), 1613-1616.
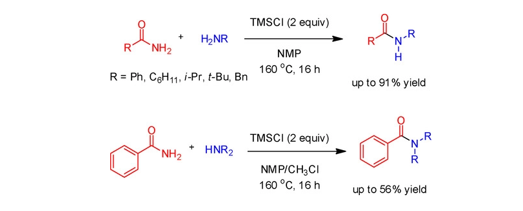

124. Cho, E.; Chen, Z.; Lee, J.; Lee, S.; Lee, T.-H. “PSTP-3,5-Me Inhibits Osteoclast Dierentiation and Bone Resorption” Molecules 2019, 24(18), 3346-3347.
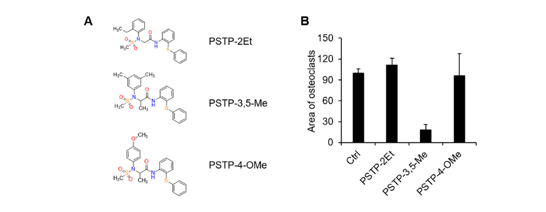

123. Idris, M. A.; Kim,
M.; Kim, J. G.; Lee, S.* “Palladium-catalyzed
decarboxylative aminocarbonylation with alkynoic acid and tertiary amine for
the synthesis of alkynyl amide” Tetrahedron 2019, 75 (31), 4130-4137.
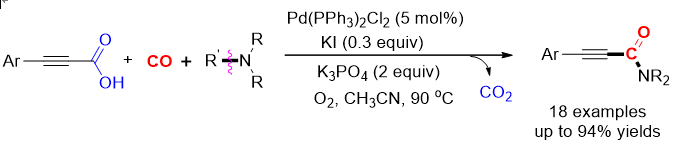

122. Han, S.; Kim,
H.-S.; Zhang, M.; Xia, Y.; Lee, S.* “Ni/Cu-Catalyzed
Decarboxylative Addition of Alkynoic Acids to Terminal Alkynes for the
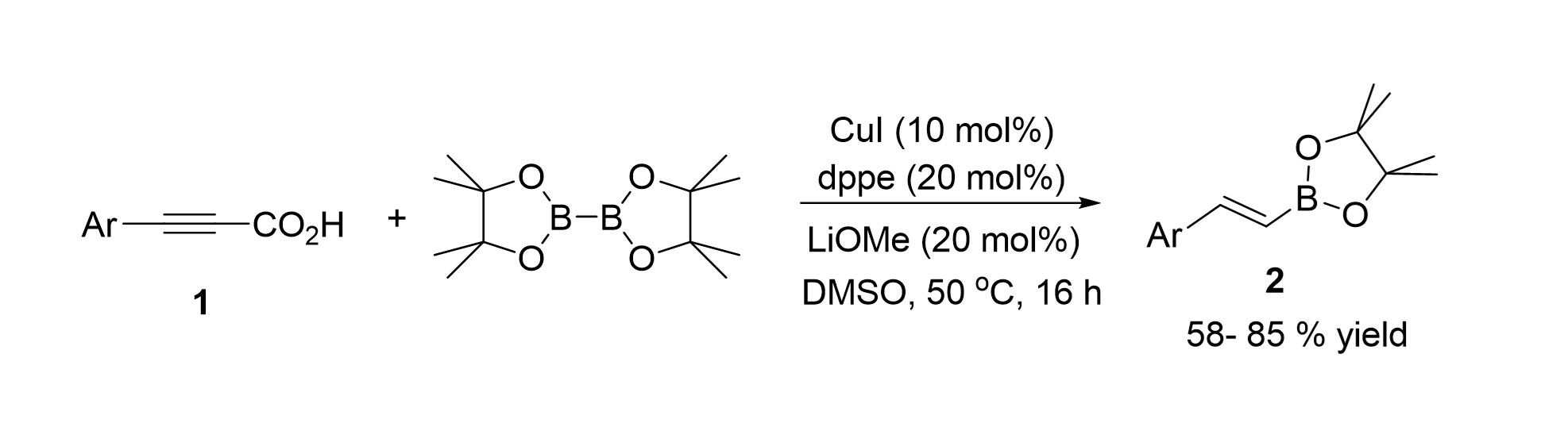
Synthesis of gem-1,3-Enynes” Org. Lett. 2019, 21(14), 5426-5431.
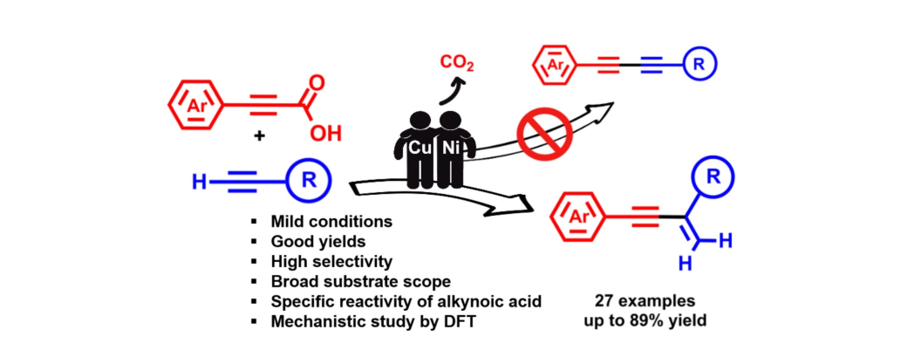

121. Jang, M.; Park, K.; Lim, J.; Oh, J.; Lee, S.* “Decarboxylative Heck-Type Reactions of Thioacrylic Acid with Aryl Bromides” Bull. Korean Chem. Soc. 2019, 40(6) 487-488.
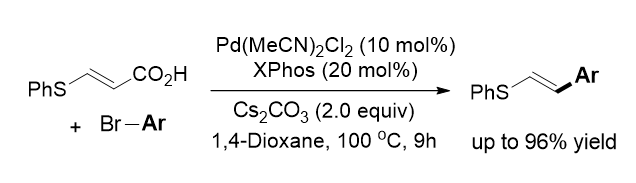

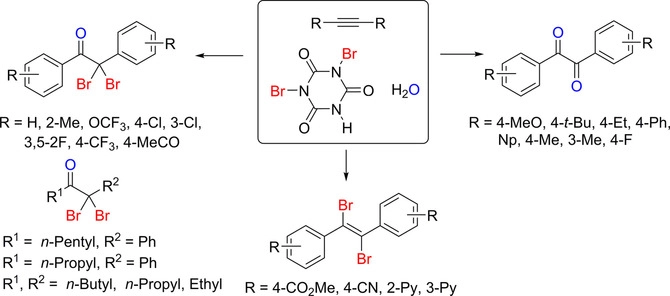
119. Cho, E.;
Jayaraman, A.; Lee, J.; Ko, K. C.; Lee,
S.* “Substituent Effect in the Synthesis of α,α‐Dibromoketones,
1,2‐Dibromalkenes, and 1,2‐Diketones from the Reaction of Alkynes and Dibromoisocyanuric
Acid” Adv. Synth. Catal. 2019, 361(8), 1846-1858.

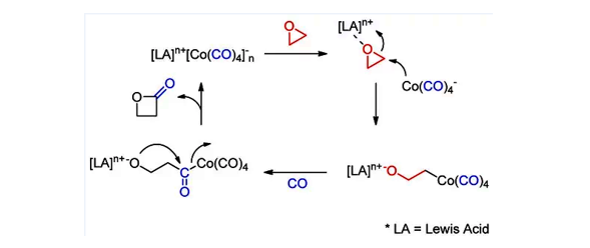
118. Baral, E. R.; Kim,
D.; Lee, S.*; Park, M. H.*; Kim, J. G.* “Tin(IV)-Porphyrin Tetracarbonyl
Cobaltate: An Efficient Catalyst for the Carbonylation of Epoxides” Catalysts 2019, 9(4) 311-323.



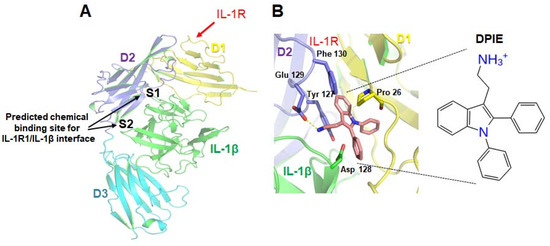
117. Ahn, S.-H.; Lee,
J.-K.; Kim, N. D.; Kim, S.-H.; Lee, S.;
Jung, S.; Chay, K.-O.; Lee, T.-H. “DPIE
[2-(1,2-diphenyl-1H-indol-3-yl)ethanamine] Augments Pro-Inflammatory Cytokine
Production in IL-1β-Stimulated Primary Human Oral Cells” Int. J. Mol. Sci.2018, 19(7), 1835

116. Yu, S. Shin, T.;
Zhang, M.; Xia, Y.; Kim, H.; Lee, S.*“Nickel/Briphos-Catalyzed Direct Transamidation of Unactivated Secondary Amides
Using Trimethylsilyl Chloride” Org. Lett. 2018, 20(23), 7563-7566.



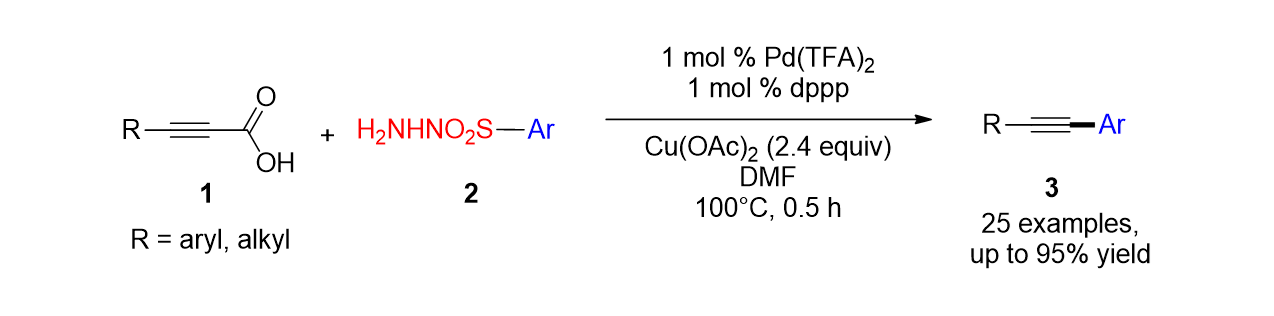

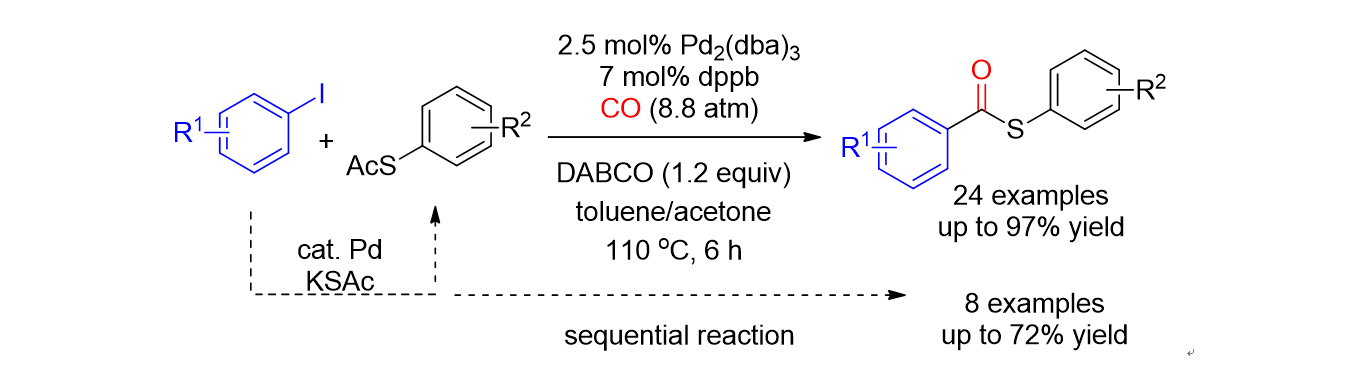

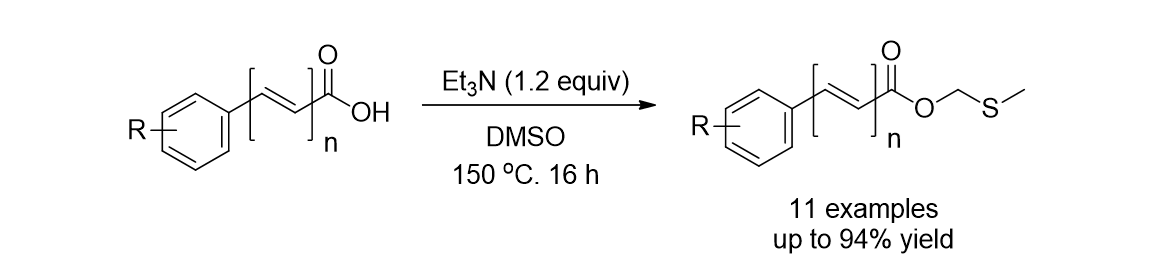
111. Yu,
S.; Nam, K. C.; Lee, S.* “Synthesis of Methylthiomethyl Esters by the Reaction
of Carboxylic Acid with Dimethylsulfoxide” Bull. Korean. Chem. Soc. 2018, 39
(7), 906-908.

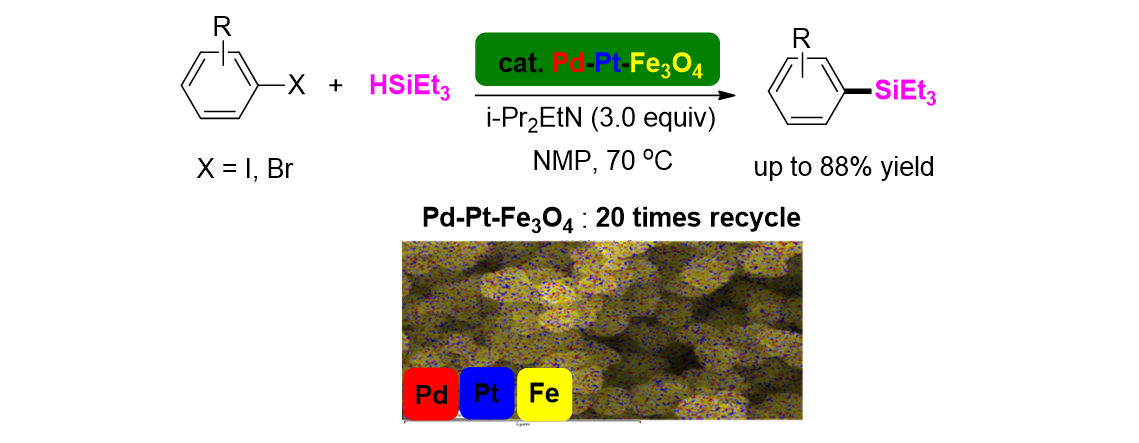

109. Ko, B. H.; Yu, S.; Song, K. H.; Lee, S.* “Continuous flow reaction system for the synthesis of 2,2,2-trichloroacetophenone derivatives and its application” Tetrahedron Lett. 2018, 59(11), 991-994.
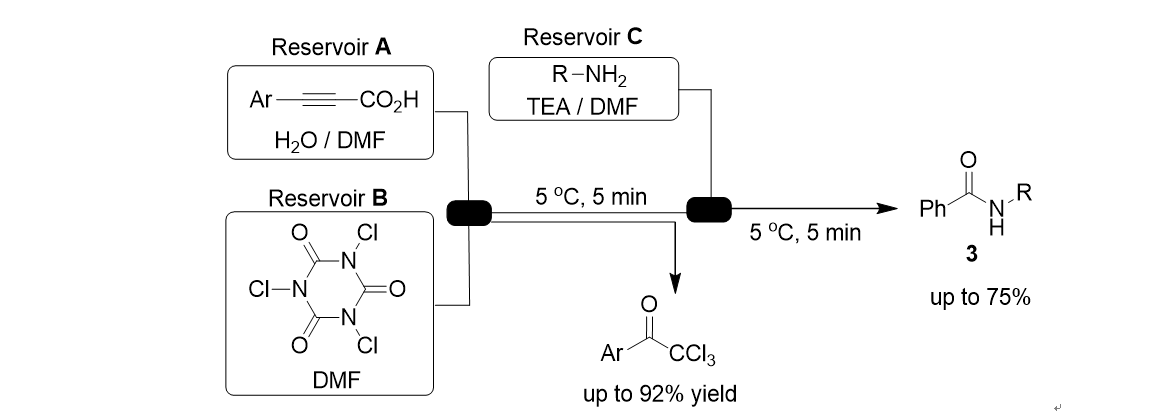

108. Cho, E.; Kim, M.; Jayaraman, A.; Kim, J.; Lee, S.* “Synthesis of α,α-Dichloroketones through Sequential Reaction of Decarboxylative Coupling and Chlorination”Eur. J. Org. Chem. 2018, 781-784. / Very Important Paper

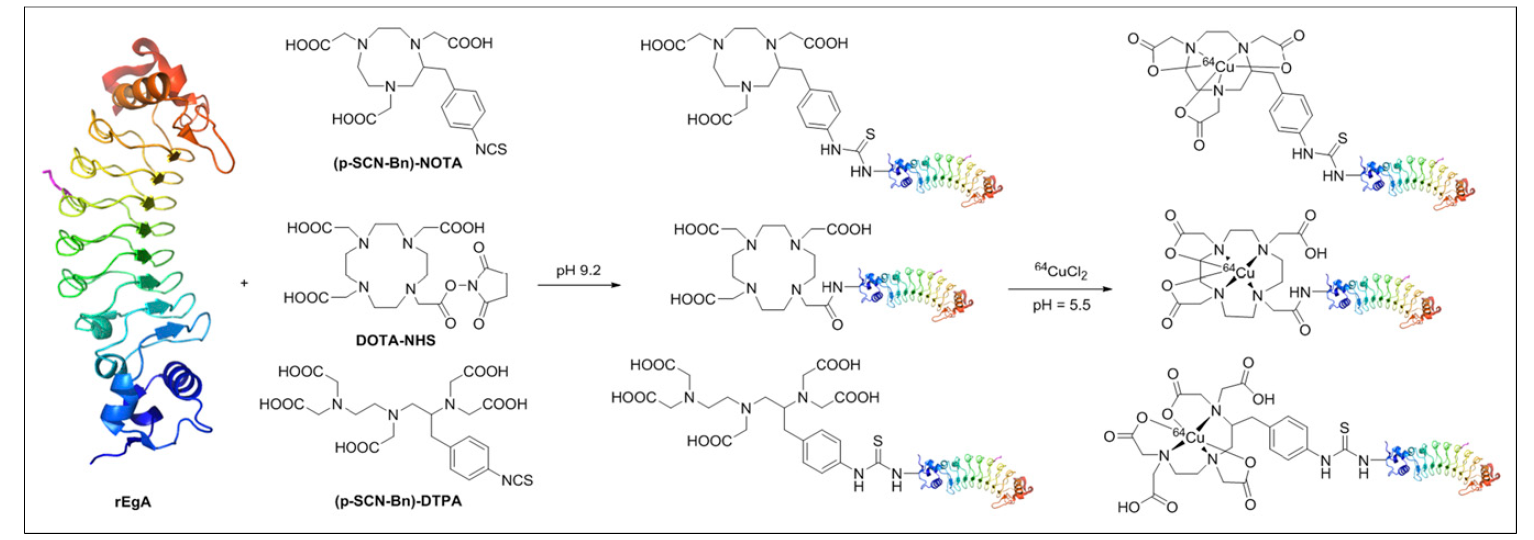
107. Pyo,
A.; Yun, M.; Kim, H. S.; Kim, T.-Y.; Lee, J.-j.; Kim, J. Y.; Lee, S.; Kwon, S.
Y.; Bom, H.-S.; Kim, H.-S.; Kim, D.-Y.; Min, J.-J. “64Cu-Labeled Repebody
Molecules for Imaging of Epidermal Growth Factor Receptor-Expressing Tumors” J. Nucl.
Med. 2018, 59(2), 340-346.

106. Jayaraman,
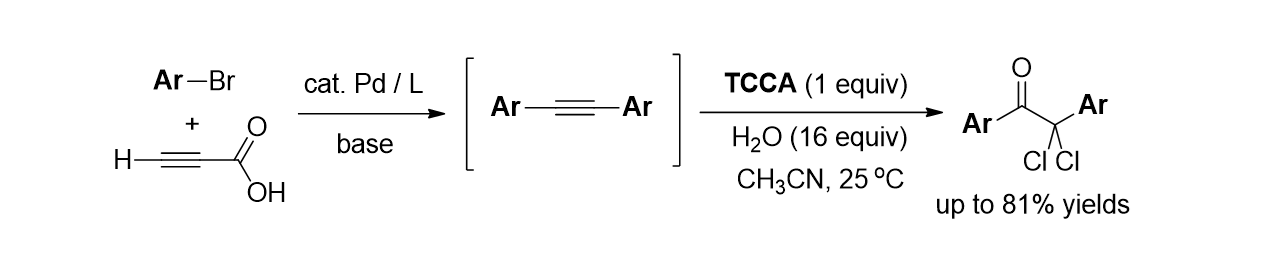
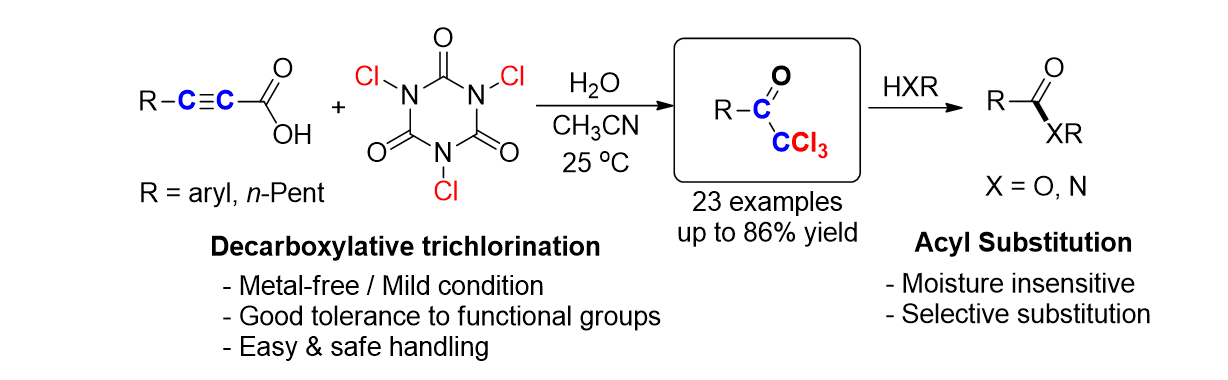
A.; Cho, E.; Irudayanathan, F. M.; Kim, J.; Lee, S.* “Metal-Free Decarboxylative
Trichlorination of Alkynyl Carboxylic Acids: Synthesis of Trichloromethyl
ketones” Adv. Synth. & Catal. 2018, 360, 130-141.




104. Raja, G. C. E.; Son, Y.; Kim, M.; Lee, S.; Oh, J. “One-pot synthesis of cinnamic anhydrides from cinnamic acids and 6-chloro-2,4-dimethoxy-sec-triazine (CDMT) at room temperature” Synth. Commun. 2017, 47 (24), 2449-2455.

103. Lee,
J.-H.; Raja, G. C. E.; Kim, J.; Nam, K.-C.; Lee, S. “Aryl Chlorides as Coupling
Partners in the Palladium-catalyzed Decarboxylative Coupling Reactions of
Propiolic Acids” B. Korean Chem. Soc.2017, 38(11), 1368-1371.

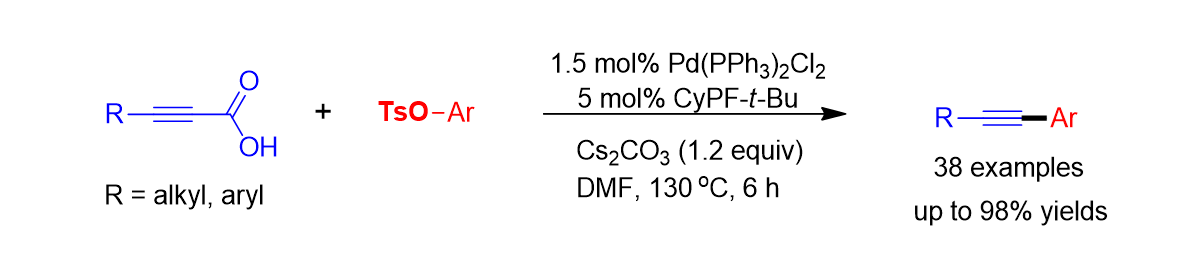
102 Yu, S.; Cho, E.; Kim, J.; Lee, S. “Palladium-Catalyzed Decarboxylative Coupling of Alkynyl Carboxylic Acids and Alkenyl Tosylates for the Synthesis of Enynones” J. Org. Chem. 2017, 82(20), 11150-11156
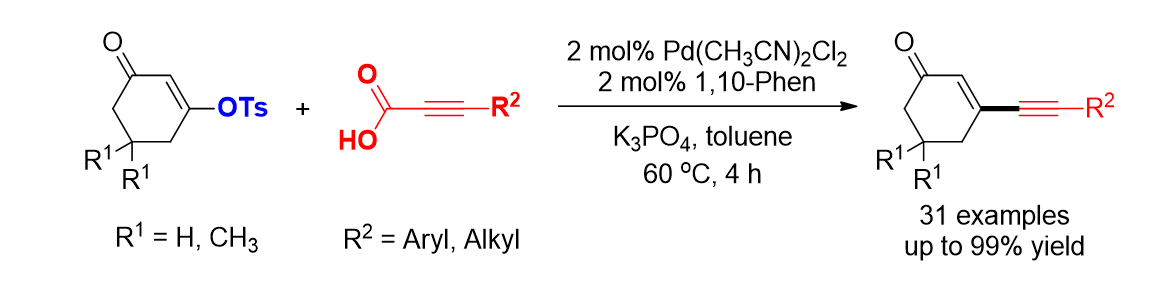

101. Park, J.; Kim, J. D.; Raja, G. C. E.; Choi, H. C.; Lee, S. "Room temperature cyclization of arylpropiolic acid anhydride: Synthesis of naphtho[2,3-c]furan-1,3-dione derivatives" Syn. Commun. 2017, 47(21), 1973-1979.
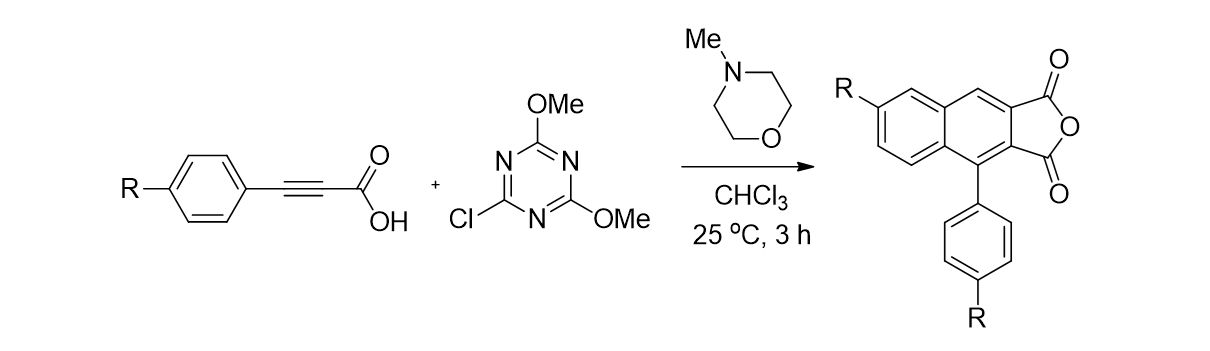


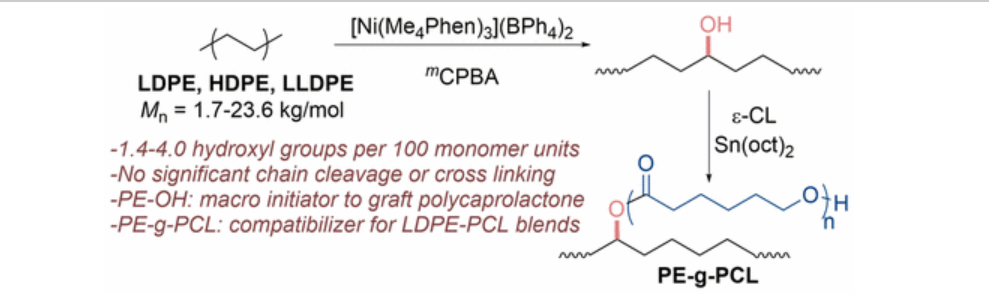
99. Bunescu, A.; Lee, S.; Li, Q.; Hartwig, J. F. “Catalytic Hydroxylation of Polyethylenes” ACS Central Sci. 2017, 3(8), 895-903.

98. Kim,
H.-S.; Kim, J. D.; Choi, H. C.; Lee, S. “UV-Irradiation-Mediated Palladium
Nanoparticle Catalytic System: Heck and Decarboxylative coupling Reactions” Mol. Catal. 2017, 441, 21-27.
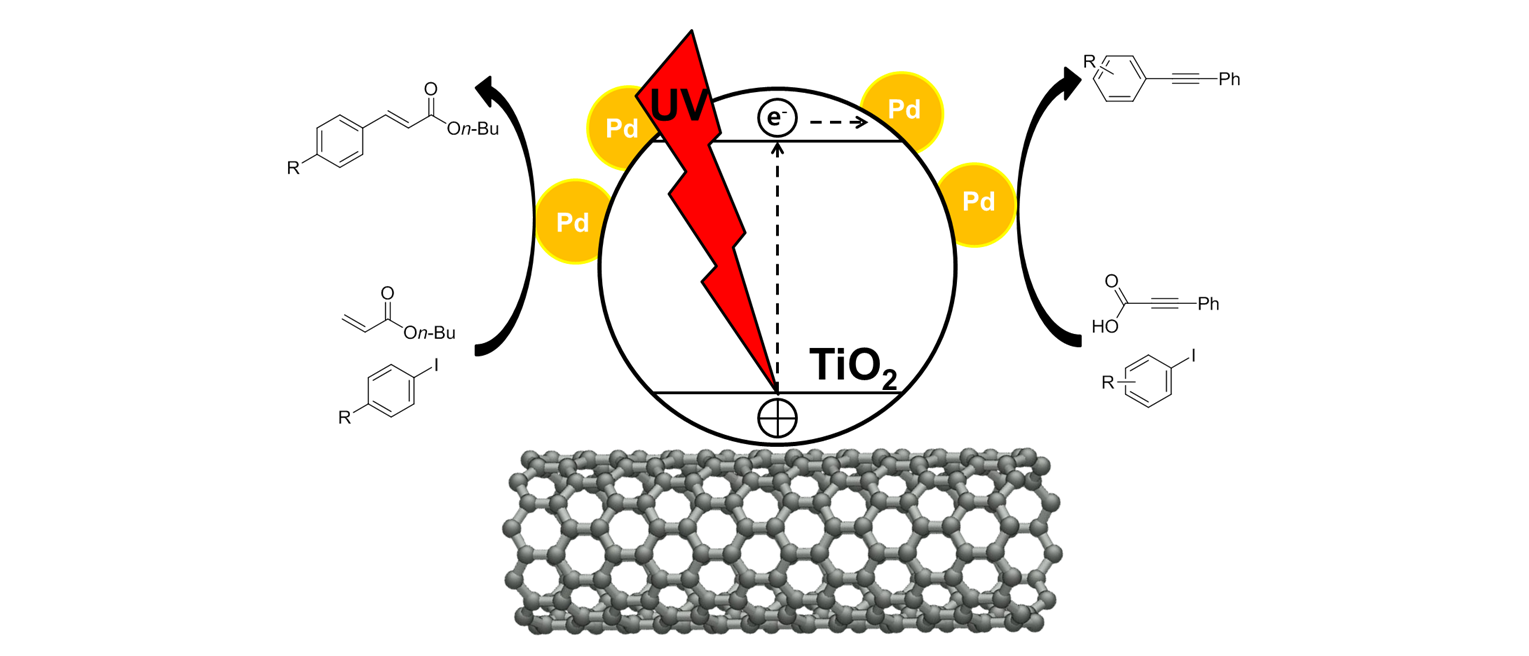

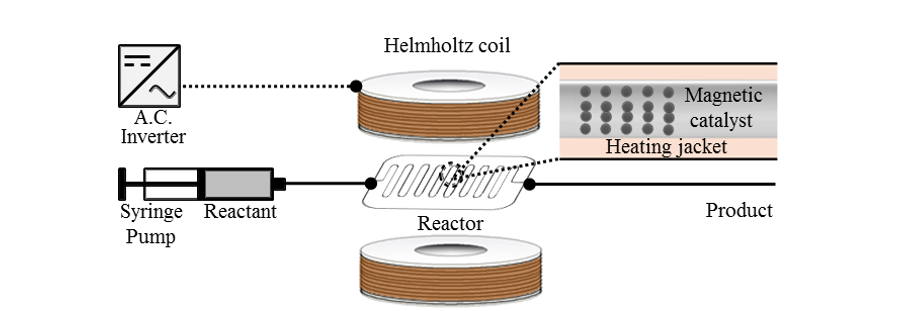
97 Kim, H. J.; Choi, J.; Choe, J.; Song, K. H.; Lee, S. “Alternating magnetic field mediated micro reaction system for palladium-catalyzed coupling reactions” RSC Adv. 2017, 7 (59), 37181-37184.

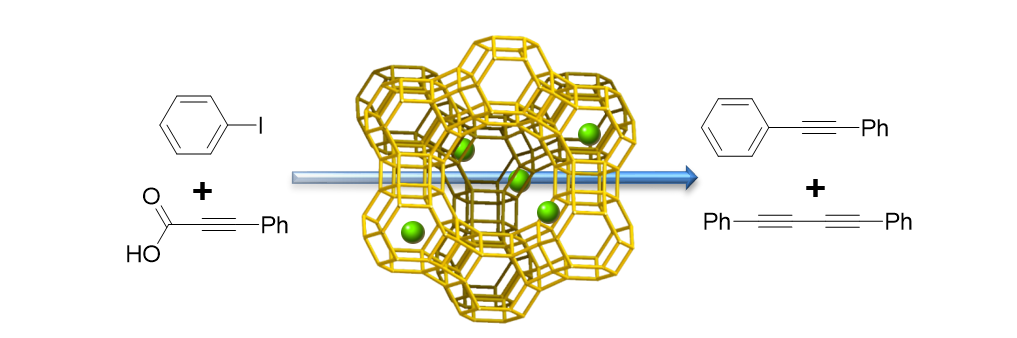
96. Park, J.; Jung, D.; Kim, H.-S.; Na, K. and Lee. S. "Zeolite-based copper catalyst for decarboxylative coupling of alkynyl carboxylic acids with aryl iodides." Catal. Commun. 2017, 99, 83-88.

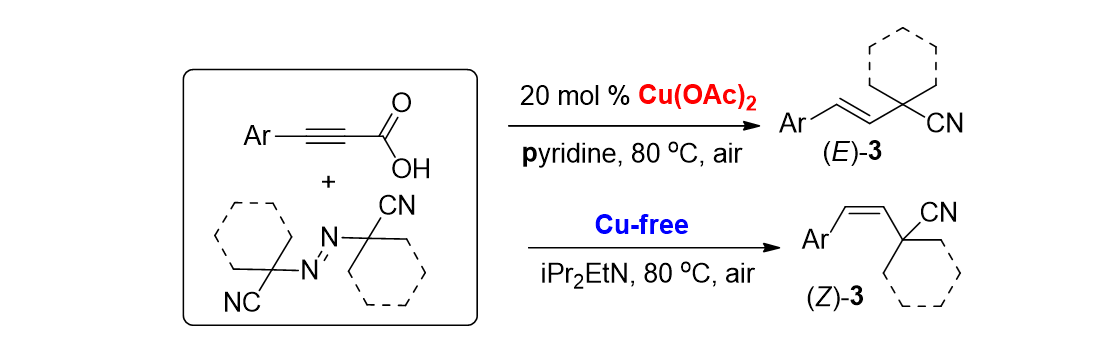
95. Irudayanathan, F. M. and Lee, S. "Selective Synthesis of (E)- and (Z)-Allyl Nitriles via Decarboxylative Reactions of Alkynyl Carboxylic Acids with Azobis(alkylcarbonitriles)" Org. Lett. 2017, 19(9), 2318-2321.

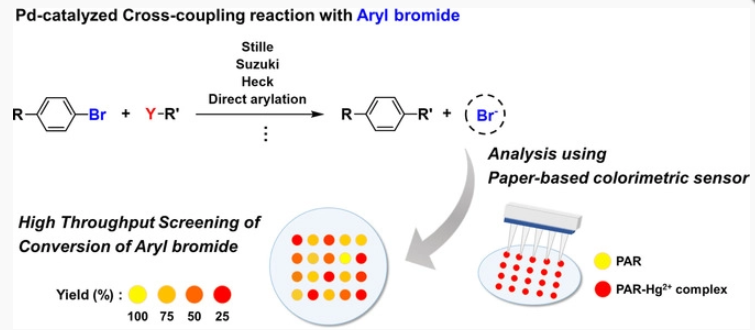
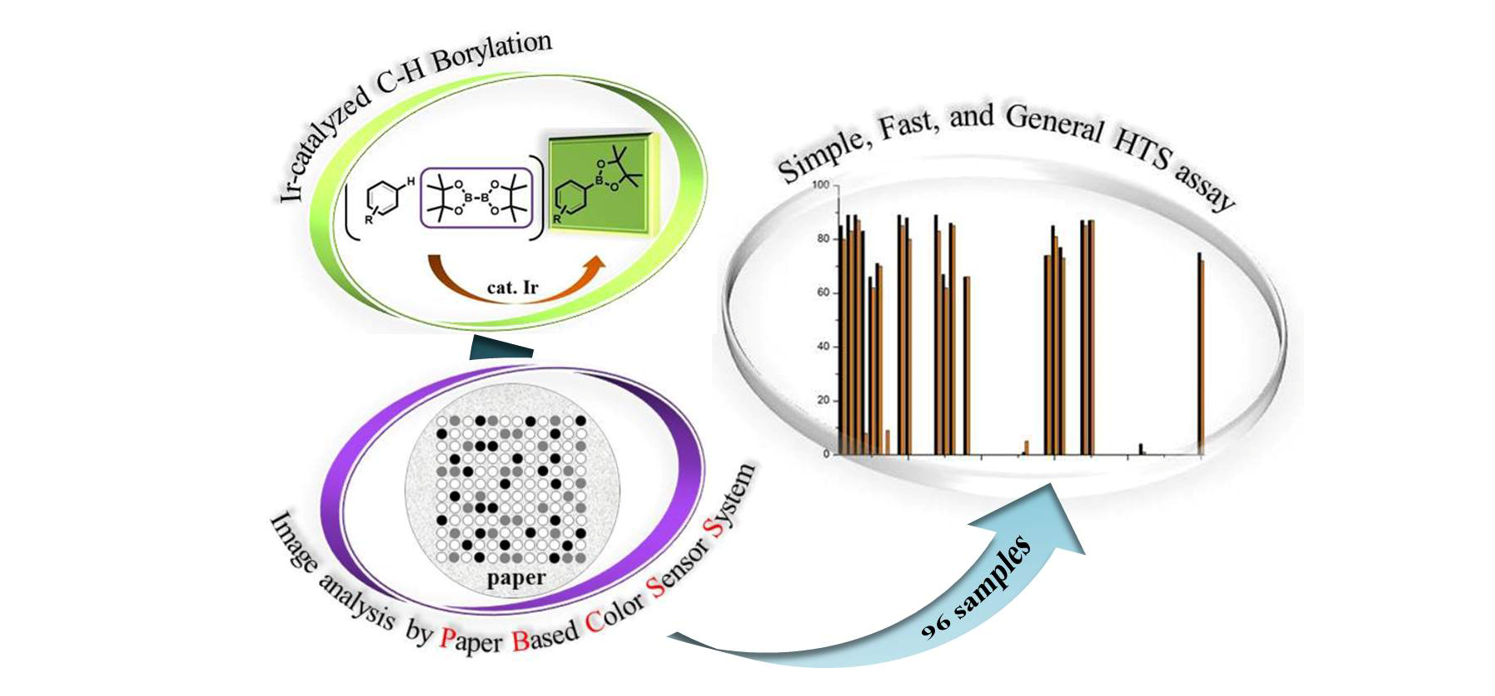
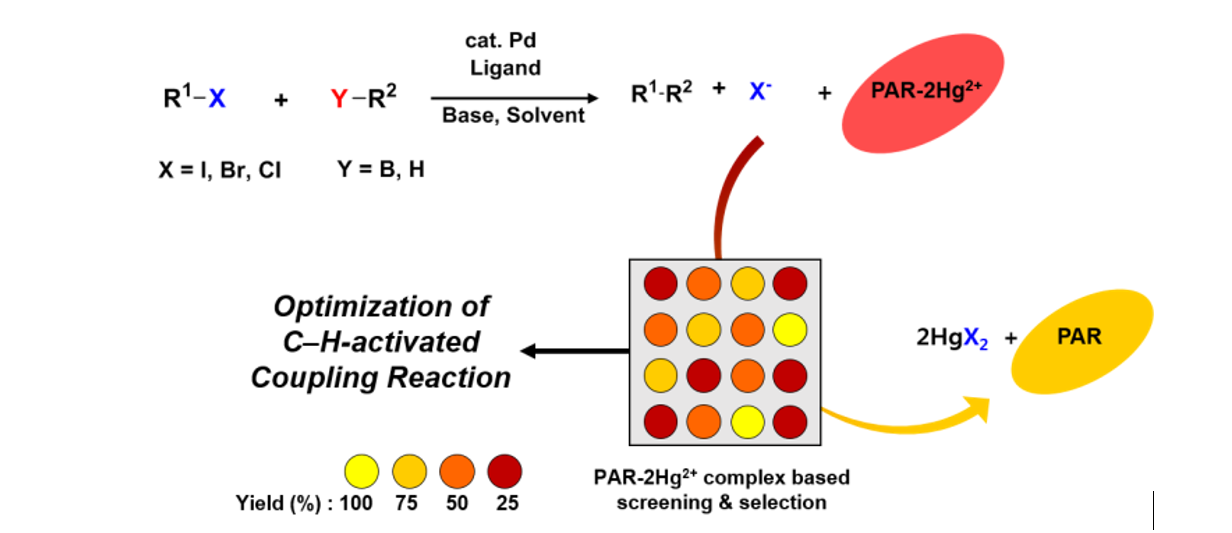
94. Kim, H.-S.; Eom, M. S.; Han, M. S. and Lee, S. "Paper-Based Colorimetric Sensor System for
High-Throughput Screening of C−H Borylation" Chem. Eur. J. 2017, 23(26), 6282-6285.

93. Son, Y.; Kim, H.-S.; Lee, J.-H.; Jang, J.; Lee, C.-F. and Lee, S. "Nickel-catalyzed decarboxylative coupling
of an alkynyl carboxylic acid with aryl iodides" Tetrahedron Lett. 2017, 58(14), 1413-1416.



92. Lee, J.-H.; Raja, G. C. E.; Son, Y.; Jang, J.; Kim, J. and Lee, S. "Nickel-catalyzed oxidative decarboxylative coupling reactions between alkynyl carboxylic acids and arylboronic acids" Tetrahedron Lett. 2016, 57(43), 4824-4828.

91. Jang, J.; Raja, G. C. E.; Lee, J.-H.; Son, Y.; Kim, J. and Lee, S. "Palladium-catalyzed decarboxylative coupling reaction with alkynyl carboxylic acids and arylsiloxanes" Tetrahedron Lett. 2016, 57(41), 4581-4584.

90. Irudayanathan, F. M.; Kim, J.; Song, K. H. and Lee, S. "Transition-Metal-Free Decarboxylative Coupling Reaction for the Synthesis of Propargyl Alcohols" Asian J. Org. Chem. 2016, 5(9), 1148-1154. (Front Cover)
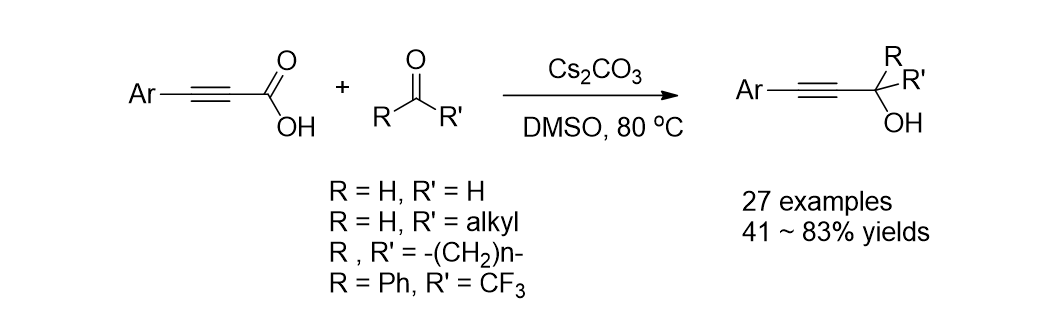

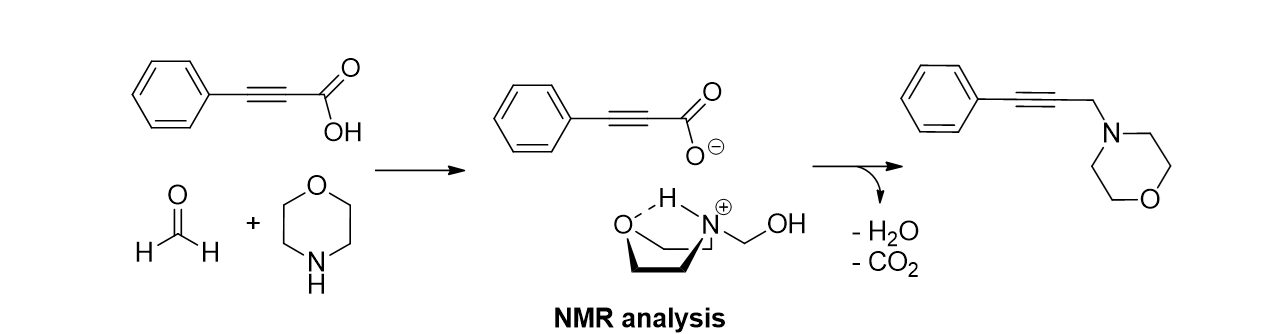
89. Lee, Y.; Park, K.; Kim, H.-S.; Kim, J.; Lee, Y. J.; Park, K. D.; Oh, J. and Lee, S. "Mechanistic studies on the metal-free decarboxylative coupling reaction for synthesis of propargylamines by NMR" Arkivoc 2016, v(5), 1-12.

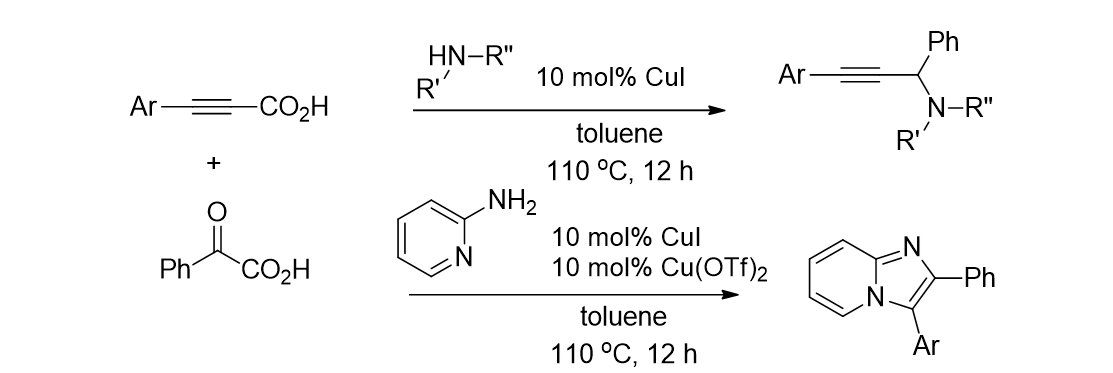

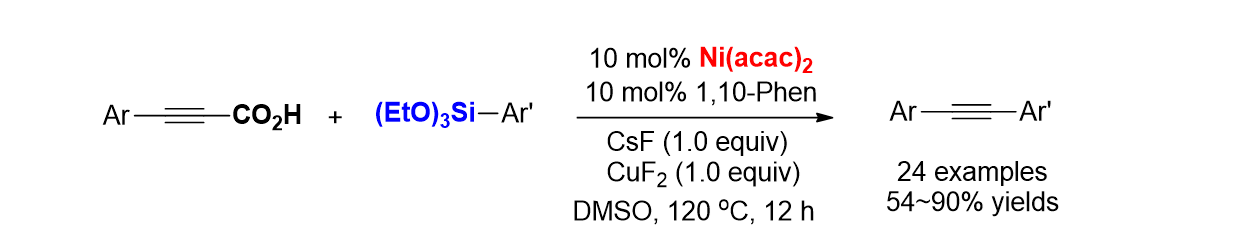




83. Lee, S.; Kim, H.-S.; Min, H.; Pyo, A. “One-pot synthesis of benzoylacetonitriles through sequential Pd-catalyzed carbonylation and decarboxylation” Tetrahedron Lett. 2016, 57(2), 239-242.

82. Lim, J.;
Choi, J.; Kim, H.-S.; Kim, I. S.; Nam, K. C.; Kim, J.; Lee, S. “Synthesis of
Terminal Allenes via a Copper-Catalyzed Decarboxylative Coupling Reaction of
Alkynyl Carboxylic Acids” J. Org. Chem.2016, 81(1), 303-308.


81. Choi,
J.; Park, K.; Lim, J.; Jung, H. M.; Lee,
S. “Copper-Catalyzed Synthesis of Amino-Substituted Polycyclic Aromatic
Hydrocarbons by the Sequential Reaction between Aryl Alkynyl Carboxylic Acids
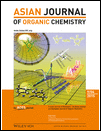
and Amines” Asian J. Org. Chem. 2015, 4, 969-974.
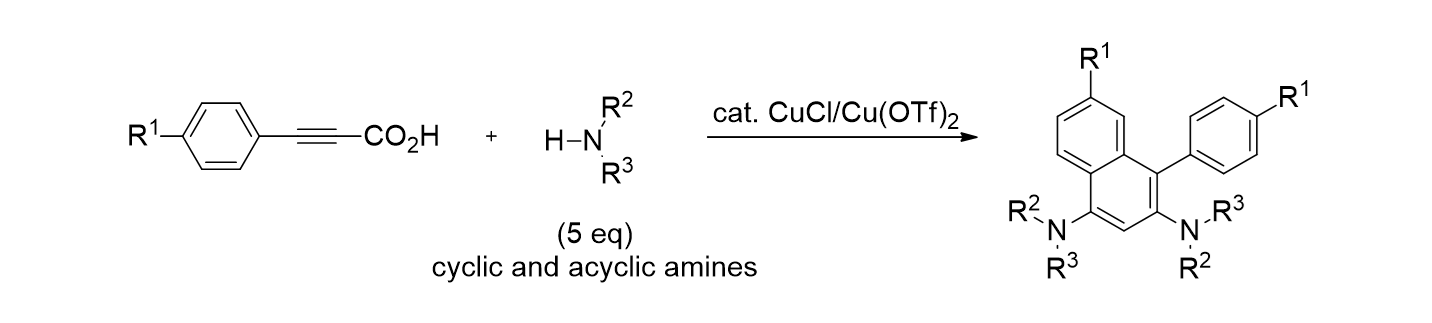

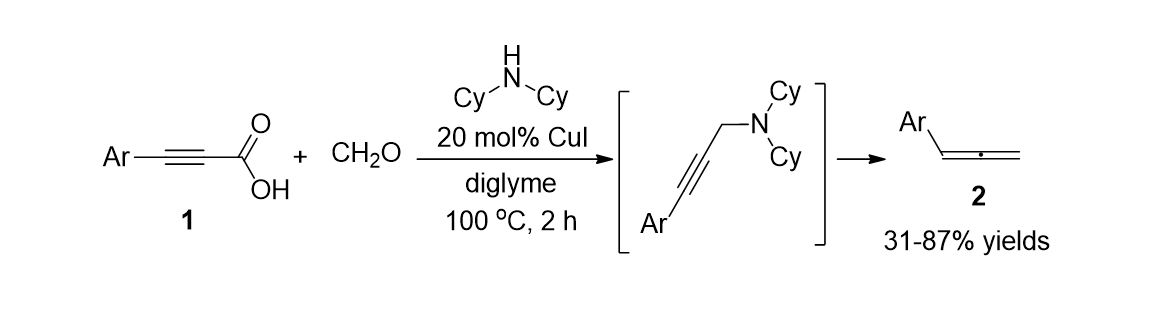
80. Jung, B.; Park, K.; Song, K. H.; Lee, S.“Continuous flow reactions in water for the synthesis of propargylamines via a metal-free decarboxylative coupling reaction” Teterahedron Lett. 2015, 56(32), 4697-4700.
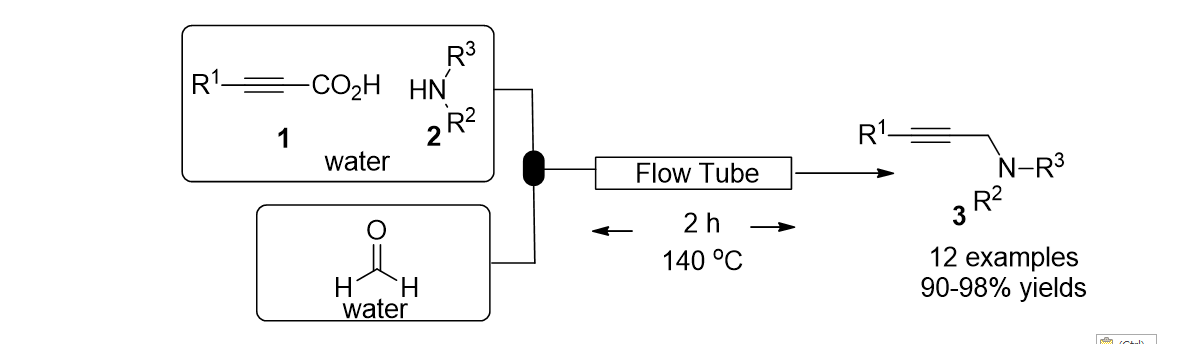



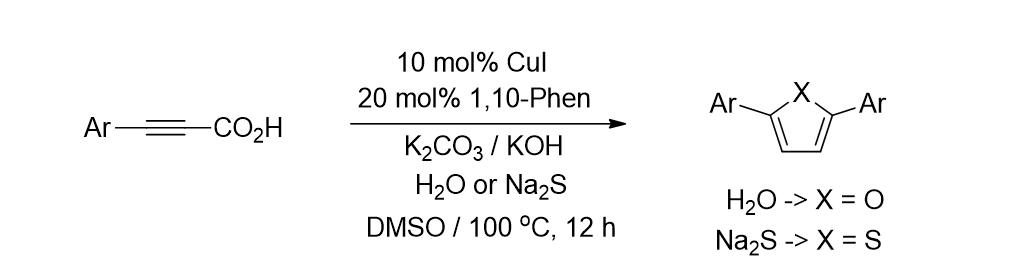
78. Irudayanathan, F. M.; G. Charles, E. R.; Lee, S. "Copper- catalyzed direct synthesis of furans and thiophenes via decarboxylative coupling of alkynyl carboxylic acids with H2O or Na2S" Tetrahedron 2015, 71(26-27), 4418–4425.

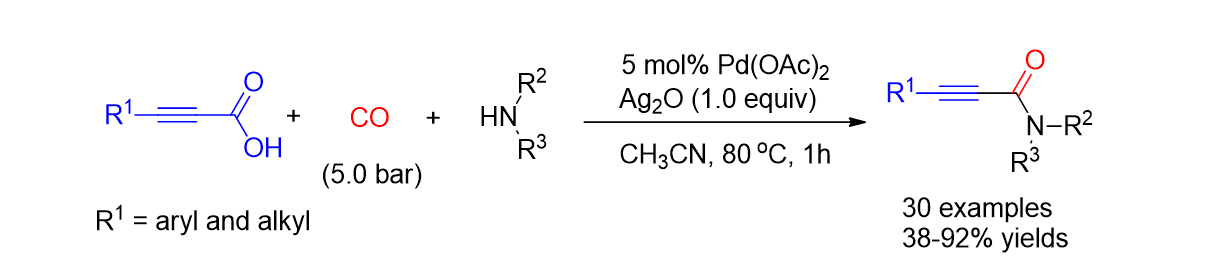
77. Hwang, J.; Choi, J.; Park, K.; Kim, W.; Song, K. H.; Lee, S. "Palladium- Catalyzed Oxidative Aminocarbonylation by Decarboxylative Coupling: Synthesis of Alkynyl Amides" Eur. J. Org. Chem. 2015, 10, 2235-2243.

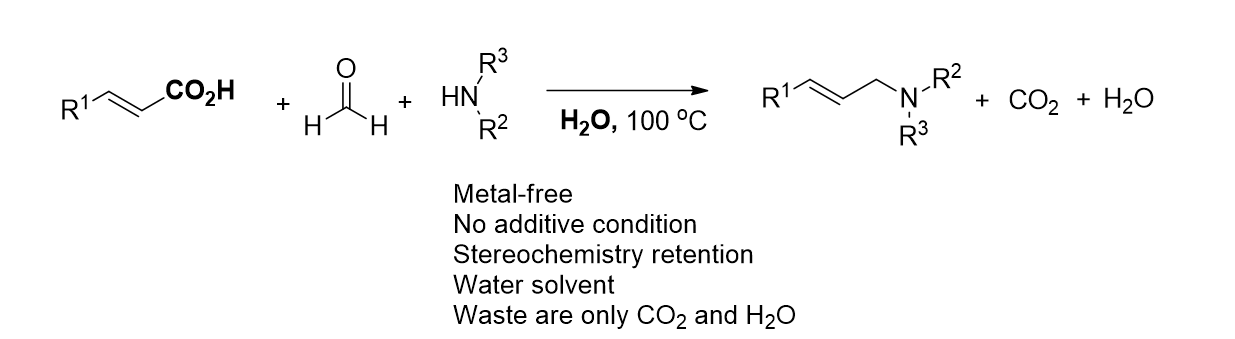
76. Park, K.; Lee, S. "Additive-Free Decarboxylative Coupling of Cinnamic Acid Derivatives in Water: Synthesis of Allyl Amines" Org. Lett. 2015, 17(5), 1300-1303.

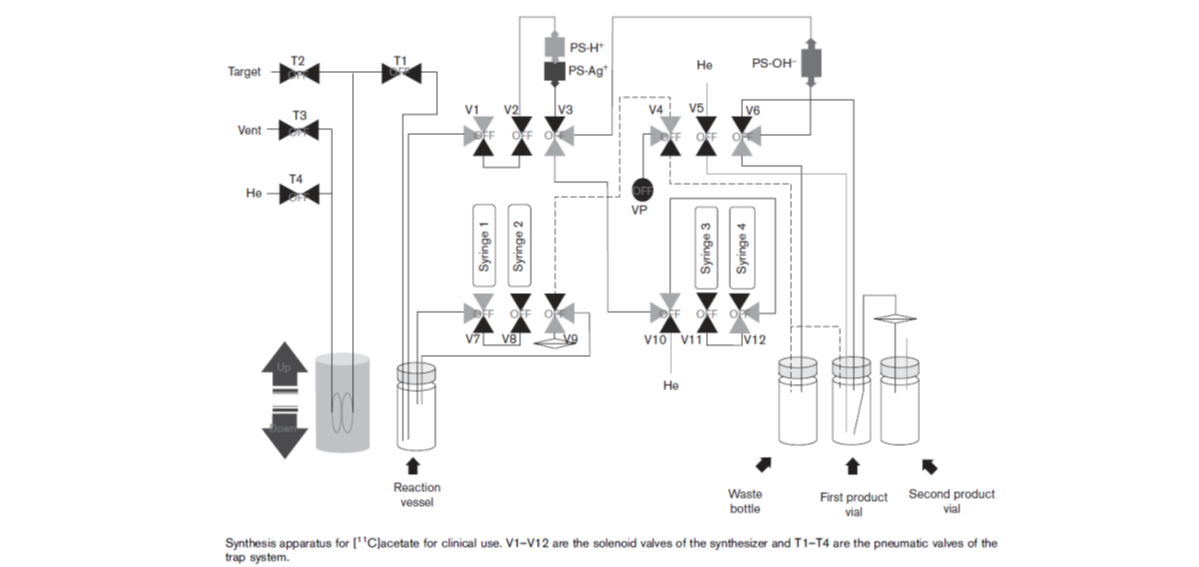
75. Jang, H. Y.; Kwon, S. Y.; Pyo, A.; Hur, M. G.; Kim, S. W.; Park, J.; Kim, H.; Yang, S. D.; Lee, S.; Kim, D.; Min, J. "In- house development of an optimized synthetic module for routine [11C] acetate production" Nucl Med Commun 2015, 36(1), 102-106.



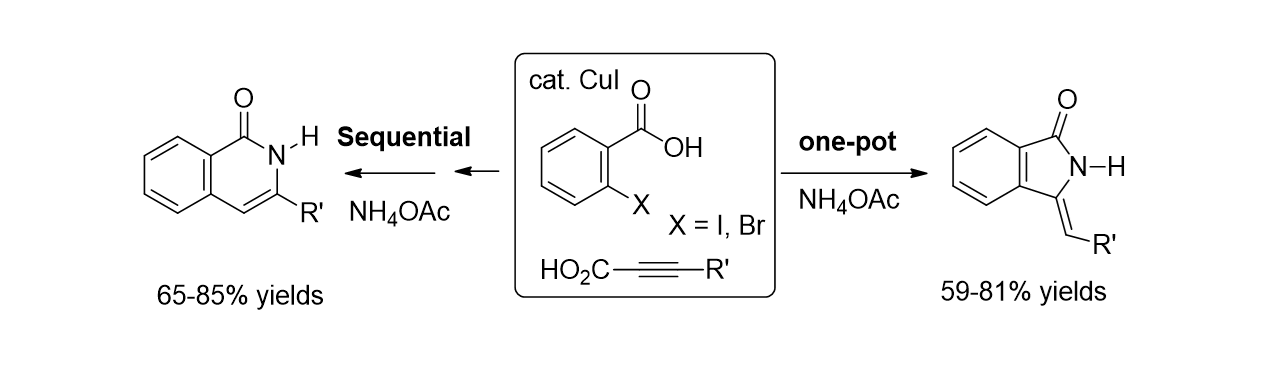
74. Irudayanathan, F. M.; Noh, J.; Choi, J.; Lee, S. "Copper- Catalyzed Selective Synthesis of Isoindolin- 1- ones and Isoquinolin- 1- ones from the Three- Component Coupling of 2- Halobenzoic Acid, Alkynylcarboxylic Acid and Ammonium Acetate" Adv. Synth. Catal. 2014, 356(16), 3433-3442.

73. Lim, J.; Park, K.; Byeun, A.; Lee, S. "Copper- catalyzed decarboxylative coupling reactions for the synthesis of propargyl amines" Tetrahedron Lett. 2014, 55(35), 4875-4878.

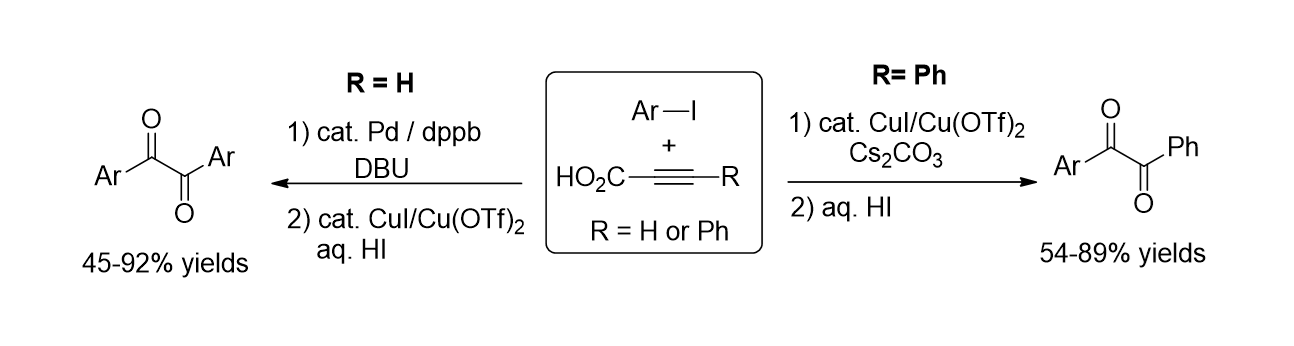
72. Min, H.; Palani, T.; Park, K.; Hwang, J.; Lee, S. "Copper- Catalyzed Direct Synthesis of Diaryl 1, 2- Diketones from Aryl Iodides and Propiolic Acids" J. Org. Chem. 2014, 79(13), 6279-6285.

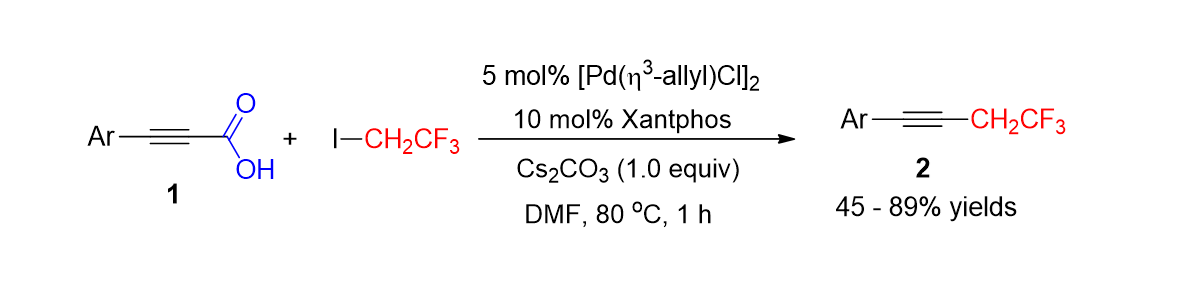
71. Hwang, J.; Park, K.; Choe, J.; Min, H.; Song, K. H.; Lee, S. "Palladium- Catalyzed Decarboxylative Trifluoroethylation of Aryl Alkynyl Carboxylic Acids" J. Org. Chem. 2014, 79(7), 3267-3271.

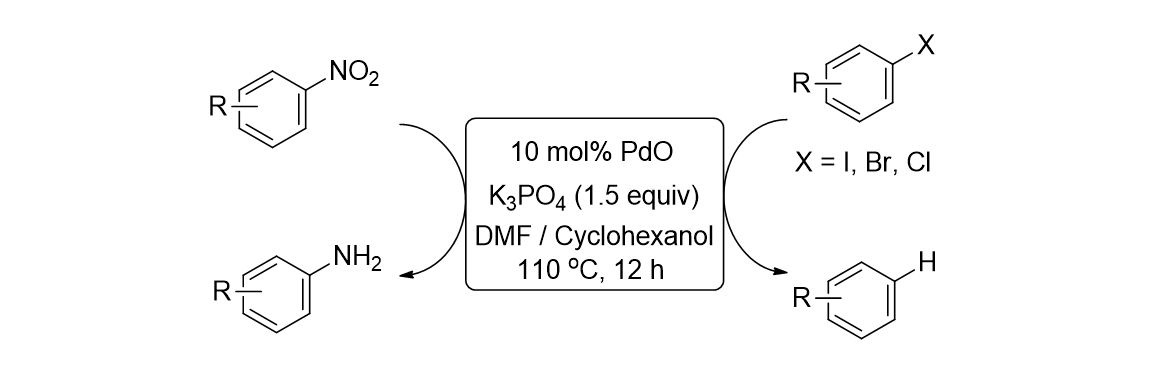
70. Min, H.; Lee, S.; Park, M.; Hwang, J.; Jung, H. M.; Lee, S. "Preparation of polymer- bound palladium catalyst and its application to the reduction of nitro arenes and the hydrodehalogenation of aryl halides" J. Organomet. Chem. 2014, 755, 7-11.

69. Kim, W.; Park, K.; Park, A.; Choe, J.; Lee, S. "Correction to Pd-Catalyzed Selective Carbonylative and Non-carbonylative Couplings of Propiolic Acid: One-Pot Synthesis of Diarylalkynones" Org. Lett. ,2014, 16(1), 325-325.




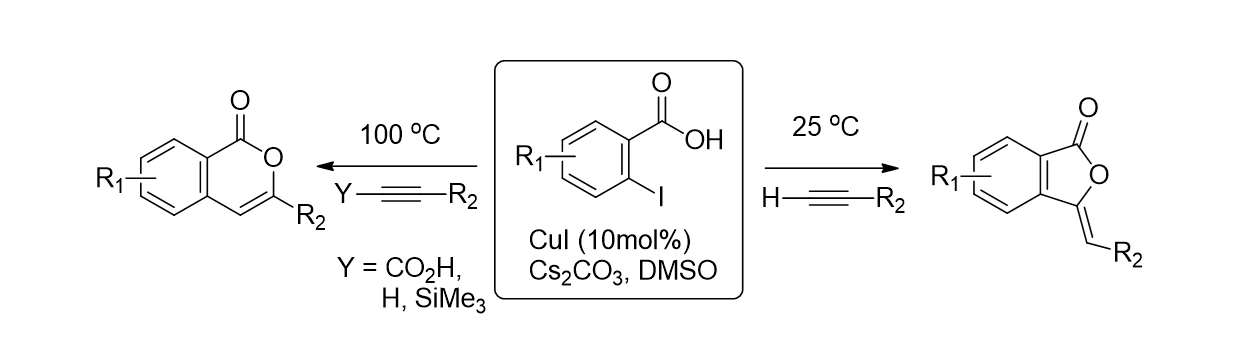
68. Kumar, M. R.; Irudayanathan, F. M.; Moon, J. H.; Lee, S. "Regioselective One- Pot Synthesis of Isocoumarins and Phthalides from 2- Iodobenzoic Acids and Alkynes by Temperature Control" Adv. Synth. Catal. 2013, 355(16), 3221-3230.

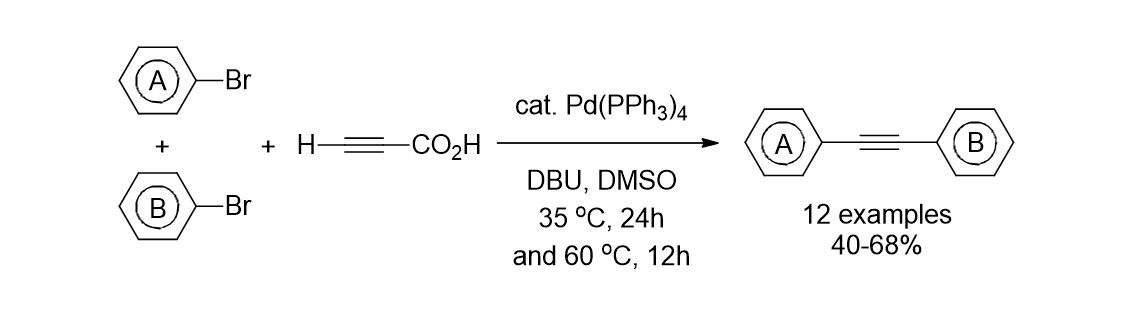
67. Park, K.; Kim, W.; Lee, S. "Efficient one- pot synthesis of the unsymmetrical diarylalkynes from two different aryl bromides and propiolic acid by using Pd(PPh3) 4 catalyst" Bull. Korean Chem. Soc. 2013, 34(10), 2859-2860.

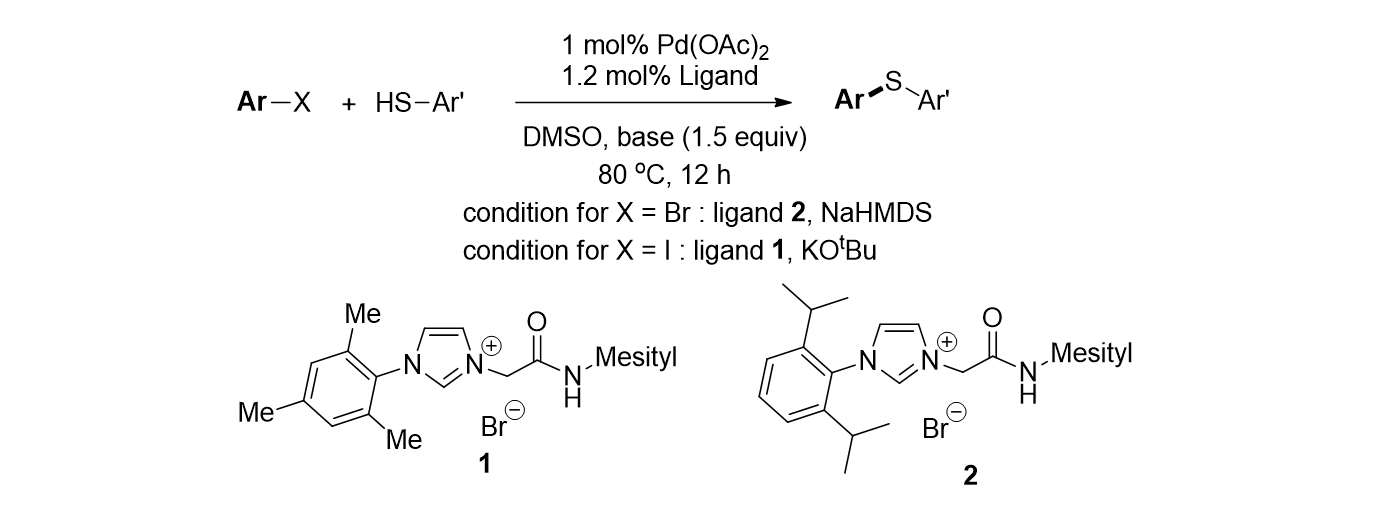
66. Byeun, A.; Baek, K.; Han, M. S.; Lee, S. "Palladium-catalyzed C-S bond formation by using N-amido imidazolium salts as ligands" Tetrahedron Lett. 2013, 54, 6712-6715.

65. Pyo, A.; Kim, S.; Kumar, M. R.; Byeun, A.; Eom, M. S.; Han, M. S.; Lee, S. “Palladium-catalyzed hydrodehalogenation of aryl halides using paraformaldehyde as the hydride source: high-throughput screening by paper-based colorimetric iodide sensor” Teterahedron Lett. 2013, 54(38), 5207-5210

64. Park, K; Lee, S. “Transition metal-catalyzed decarboxylative coupling reactions of alkynyl carboxylic acids” RSC Adv. 2013, 3(34), 14165-14182. Invited Review

63. Kim, J. D.; Pyo, A.; Park, K.; Kim, G. C.; Lee, S.; Choi, H. C. “Ligand Effect in Recycled CNT-Pd Heterogeneous Catalyst for Decarboxylative Coupling Reaction” Bull. Korean Chem. Soc. 2013, 34(7) 2099-2104.

62. Park, K.; Heo, Y.; Lee, S. “Metal-Free Decarboxylative Three-Component Coupling Reaction for the Synthesis of Propargylamines” Org. Lett. 2013, 15(13), 3322-3325.

61. Vokata, T.; Kumar, M. R.; Park, K.; Moon, J. H.; Lee, S. “Synthesis of Poly(phenylenebutadiynylenes) Using the Decarboxylative Coupling of Propiolic Acid and Aryl Iodides” Synlett 2013, 1563-1567. Synfact
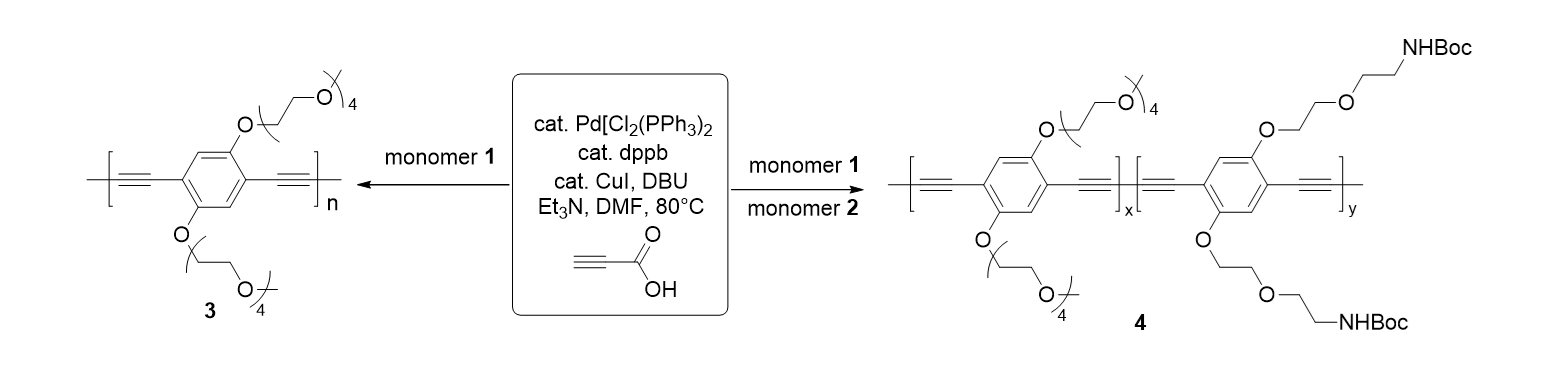

60. Palani,T.; Park, K.; Song, K. H.; Lee, S. “Palladium-Catalyzed Synthesis of (Z)-3-Arylthioacrylic Acids and Thiochromenones” Adv. Synth. Catal. 2013, 355, 1160-1168.
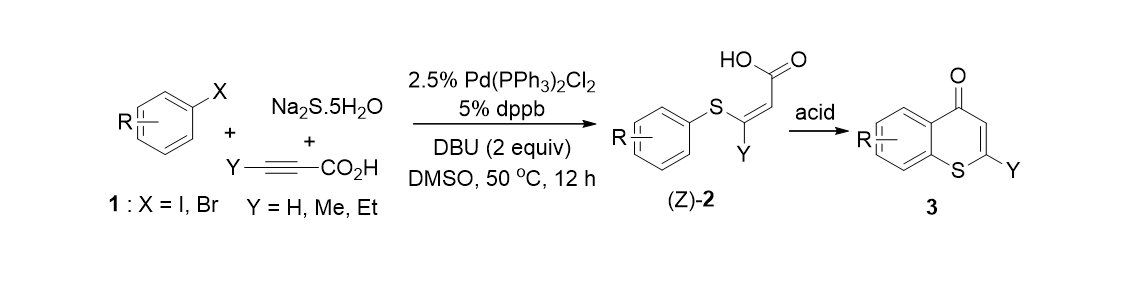

59. Kim, W.; Park, K.; Park, A.; Choe, J.; Lee, S. “Pd-Catalzyed Selective Carbonylative and Non-Carbonylative Couplings of Propiolic Acid: One-Pot Synthesis of Diaryl Alkynones” Org. Lett. 2013, 15 (7), 1654-1657.

58. Park, K.; You, J.-M.; Jeon, S.; Lee, S. “Pd(PPh3)4-Catalyzed Sonogashira Reaction for the Synthesis of Aryl Alkynyl Carboxylic Acids from Aryl Bromides at Low Temperature” Eur. J. Org. Chem. 2013, (10), 1973-1978.

57. Pyo, A.; Kim, J. D.; Choi, H. C.; Lee, S. "Ligand-free palladium-catalyzed decarboxylative coupling reactions of aryl iodides and alkynyl carboxylic acids" J. Organomet. Chem. 2013, 724, 271-274.
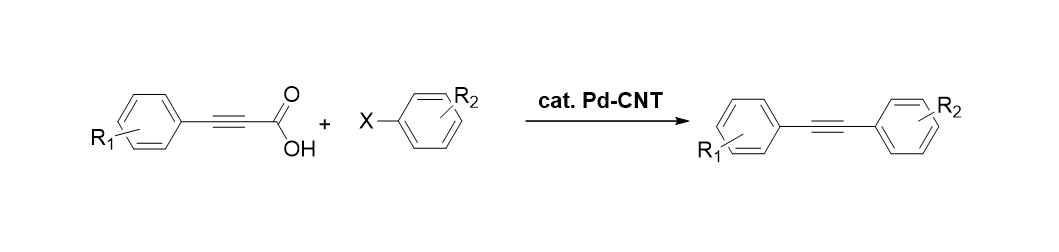

[2012]


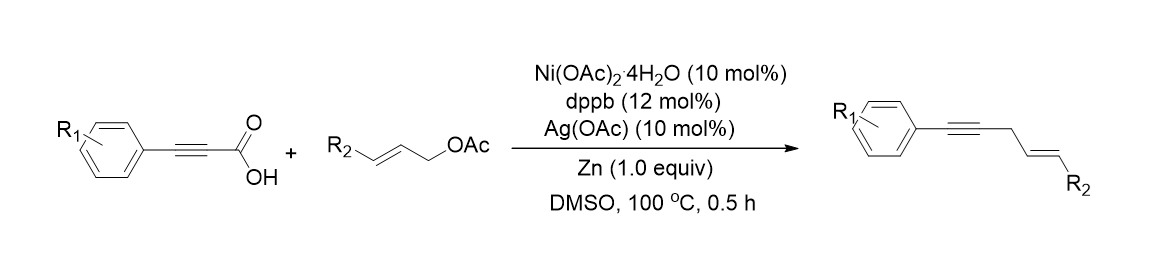
56. Choe, J.; Yang, J.; Park, K.; Palani, T.; Lee, S. "Nickel-catalyzed decarboxylative coupling reaction of alkynyl carboxylic acids and allyl acetates" Tetrahedron Lett. 2012, 53, 6908-6912.

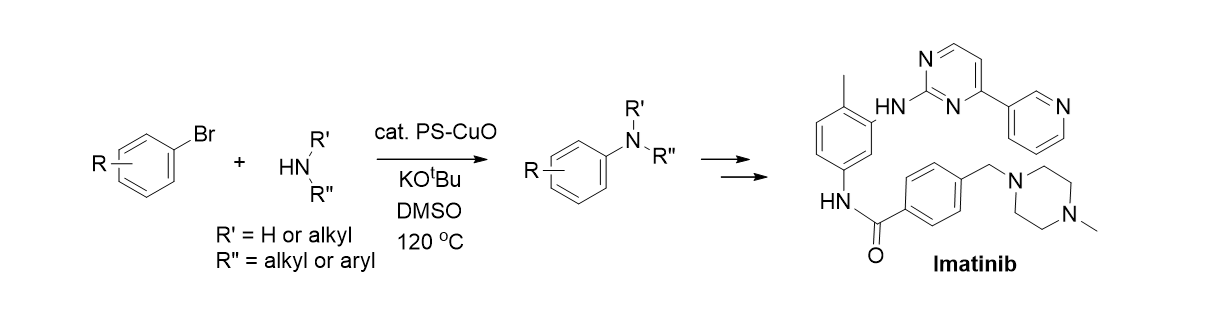
55. Heo, Y.; Hyun, D.; Kumar, M. R.; Jung, H. M.; Lee, S. "Preparation of copper(II) oxide bound on polystyrene beads and its application in the aryl aminations: synthesis of Imatinib" Tetrahedron Lett. 2012, 53, 6657-6661.- Synfact

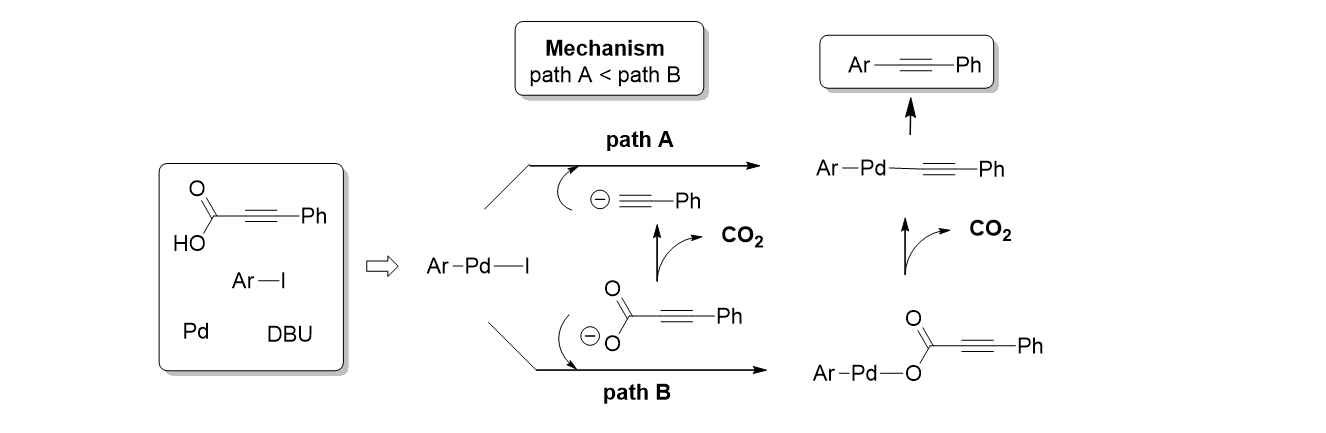
54. Pyo, A.; Kim, Y. H.; Park, K.; Kim, G. C.; Choi, H. C.; Lee, S. "Mechanistic study of palladium-catalyzed decarboxylative coupling of phenylpropiolic acid and aryl iodide" Appl. Organometal. Chem. 2012, 26, 650-654.

53. Kim, J. D.; Palani, T.; Kumar, M. R.; Lee, S.; Choi, H. C. "Preparation of reusable Ag-decorated graphene oxide catalyst for decarboxylative cycloaddition" J. Mater. Chem. 2012, 22(38), 20668-20670.
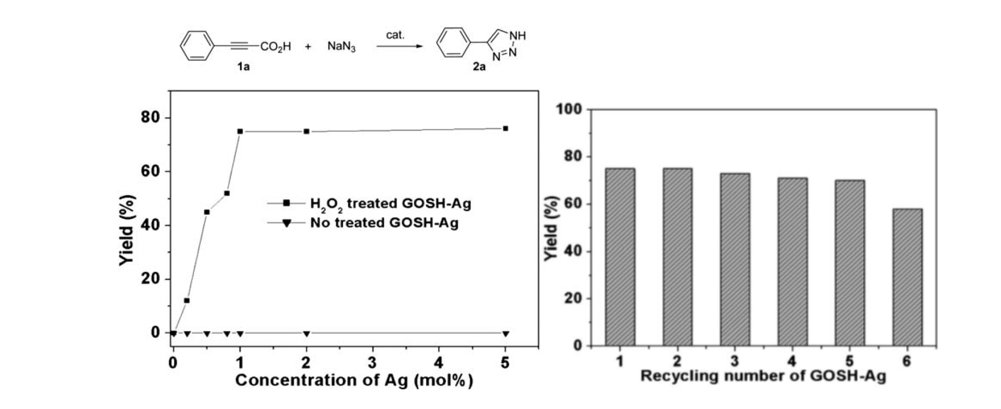

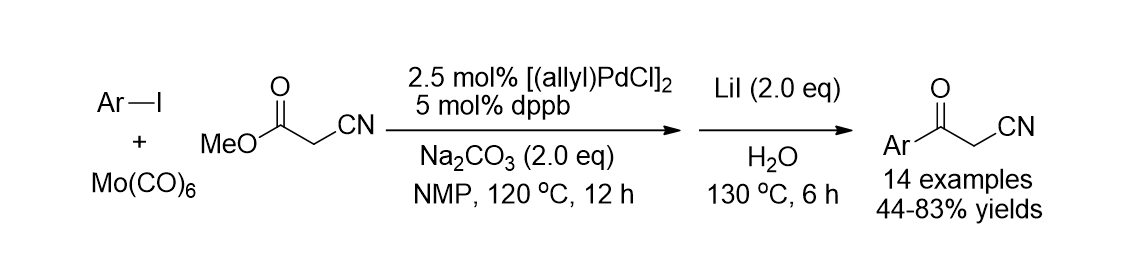
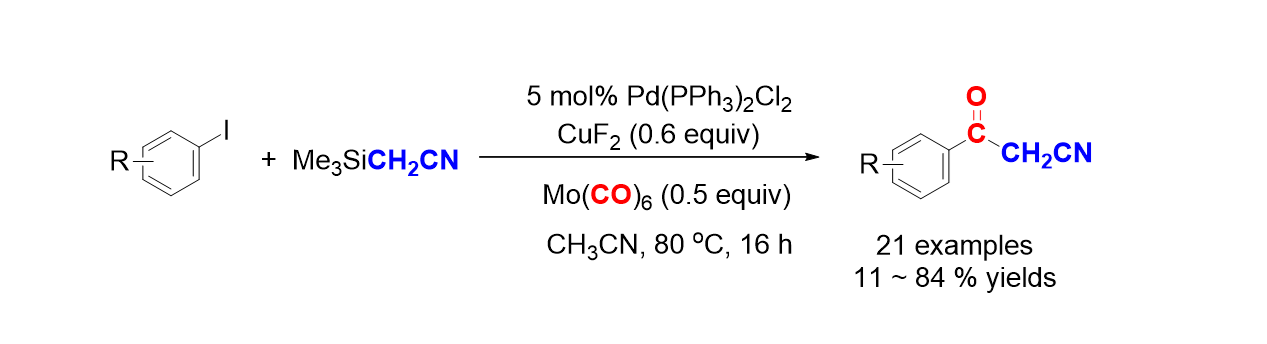
52. Pyo, A.; Park, A.; Jung, H. M.; Lee, S. "Palladium-Catalyzed Carbonylation with Mo(CO)6 for the Synthesis of Benzoylacetonitriles" Synthesis 2012, 44(18), 2885-2888.

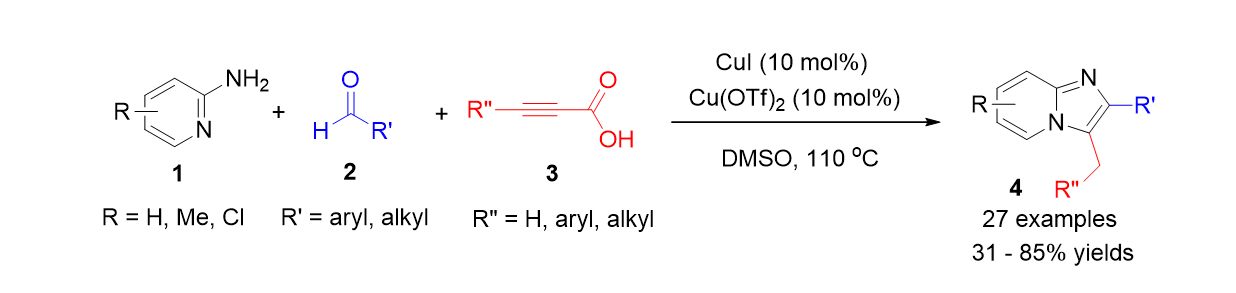
51. Palani, T.; Park, K.; Kumar, R. M.; Jung, H. M.; Lee, S. "Copper-Catalyzed Decarboxylative Three Component Reaction for the Synthesis of Imidazo[1,2-a]pyridine" Eur. J. Org. Chem. 2012, (26), 5038-5047.

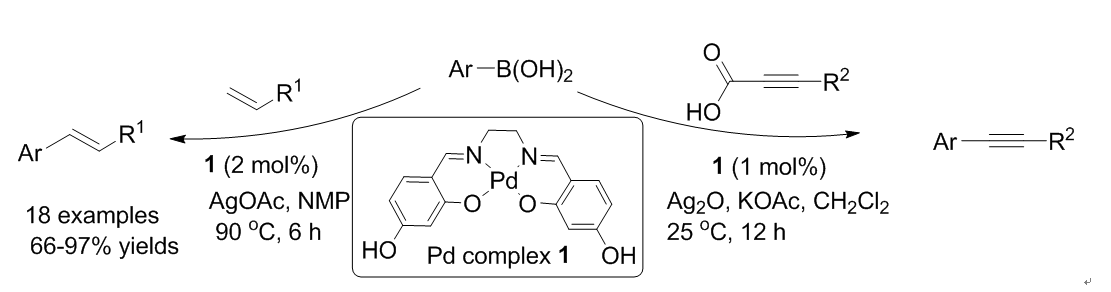
50. Heo, Y.; Kang, Y. Y.; Palani, T.; Lee, J.; Lee, S. "Synthesis, characterization of palladium hydroxysalen complex and its application in the coupling reaction of arylboronic acids: Mizoroki-Heck type reaction and decarboxylative couplings" Inorg. Chem. Commun. 2012, 23, 1-5.
49. Kim, S.: Jung, E.; Kim, M. J.; Pyo, A.; Palani, T.; Eom, M. S.; Han, M. S.; Lee, S. "Simple, Fast, and Easy Assay for Transition Metal-Catalyzed Coupling Reactions using Paper-Based Colorimetric Iodide Sensor" Chem. Commun. 2012, 48 (70), 8751-8753. - Backcover
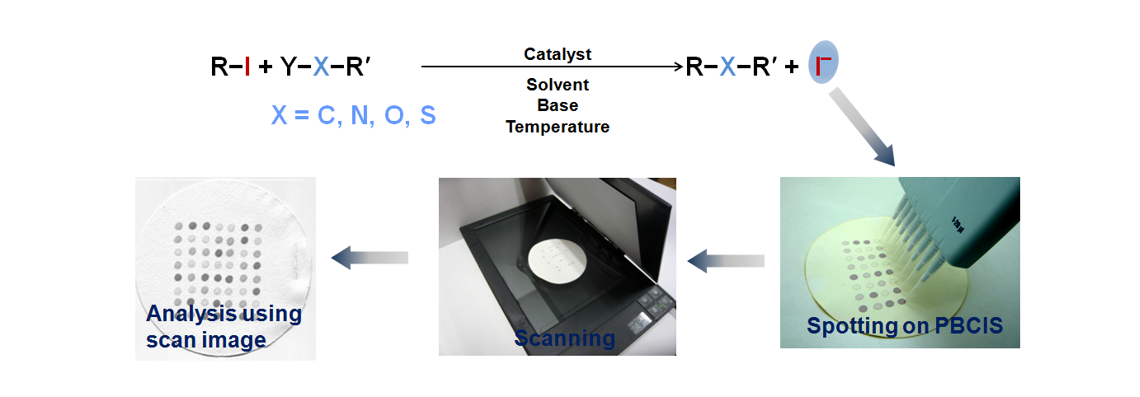

48. Park, N.; Heo, Y.; Kumar, M. R.; Kim, Y.; Lee, S."Synthesis of Benzothiazoles by Copper-Catalyzed One-Pot Three Component Reactions using Sodium Hydrosulfide as a Sulfur Surrogate" Eur. J. Org. Chem. 2012, (10), 1984-1993.
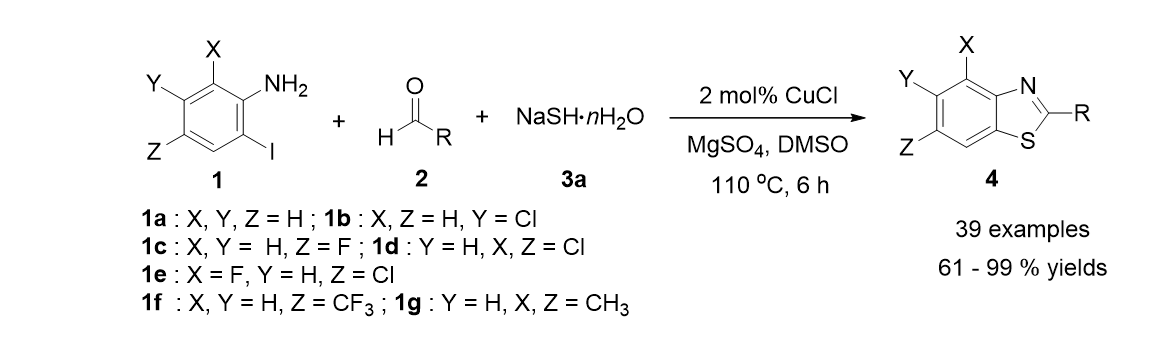

47. Park, A.; Lee, S."Synthesis of Benzoylacetonitriles from Pd-Catalyzed Carbonylation of Aryl Iodides and Trimethylsilylacetonitrile". Org. Lett. 2012, 14(4), 1118-1121.

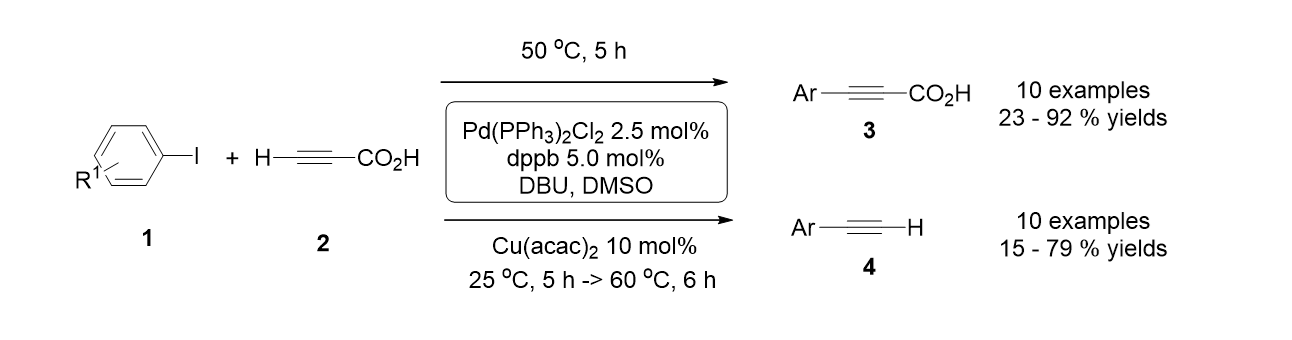
46. Park, K.; Palani, T.; Pyo, A.; Lee, S. "Synthesis of Aryl Alkynyl Carboxylic Acids and Aryl Alkynes from Propiolic Acid and Aryl Halides by Site Selective Coupling and Decarboxylation" Teterahedron Lett. 2012, 53(7), 733-737.

[2011]


45. Kim, Y.; Kumar, M. R.; Park, N.; Heo, Y.; Lee, S. "Copper-Catalyzed One-Pot Three-Component Synthesis of Benzimidazoles by Condensation and C-N Bond Formation" J. Org. Chem. 2011, 76(23), 9577-9583.- Featured Article
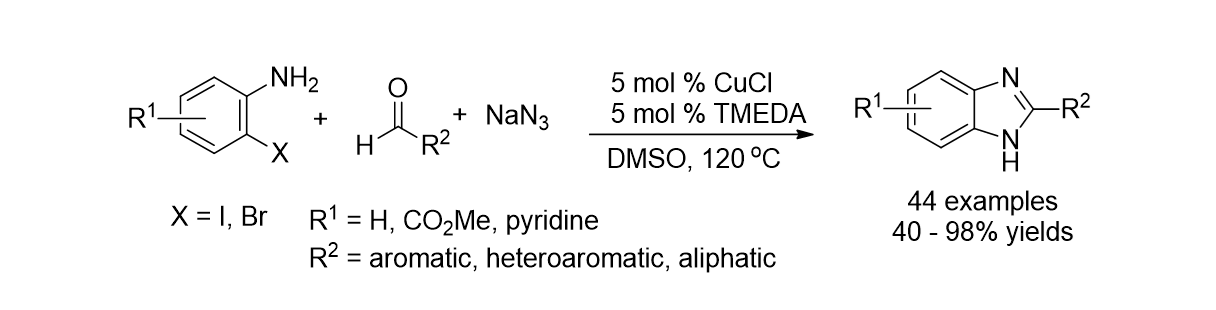

44. Yeom, J.-E.; Kumar, M. R.; Lee, S.; Lee, J.-B.; Park, H.-R. "Synthesis of Flavano-4-ol and its Spectroscopic Properties in Aqueous Solution" Bull. Korean Chem. Soc. 2011, 32(11), 4092-4094.
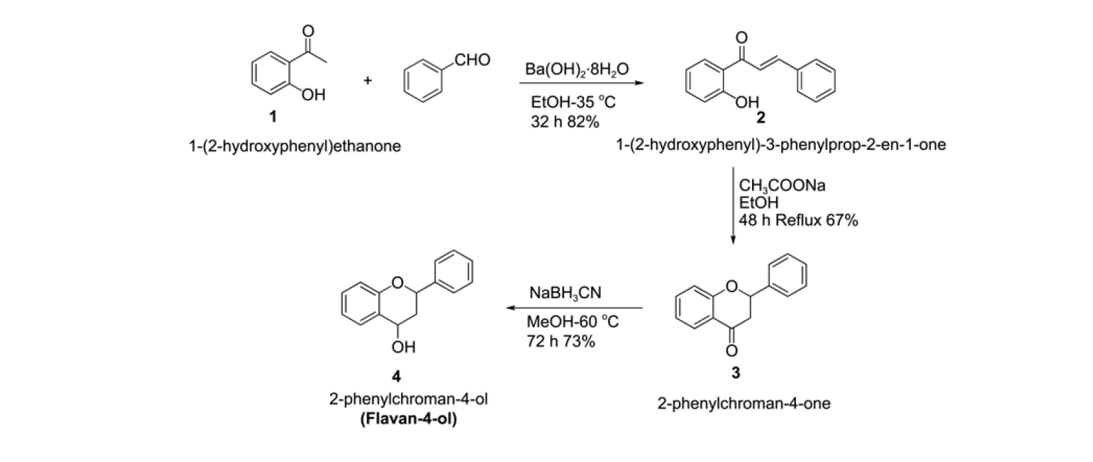

43. Lee, H. J.; Park, K.; Bae, G.; Choe, J.; Song, K. H.; Lee, S."Efficient Synthesis of Unsymmetric Diarylalkynes from Decarboxylative Coupling in a Continuous Flow Reaction System" Tetrahedron Lett. 2011, 52(39), 5064-5067.
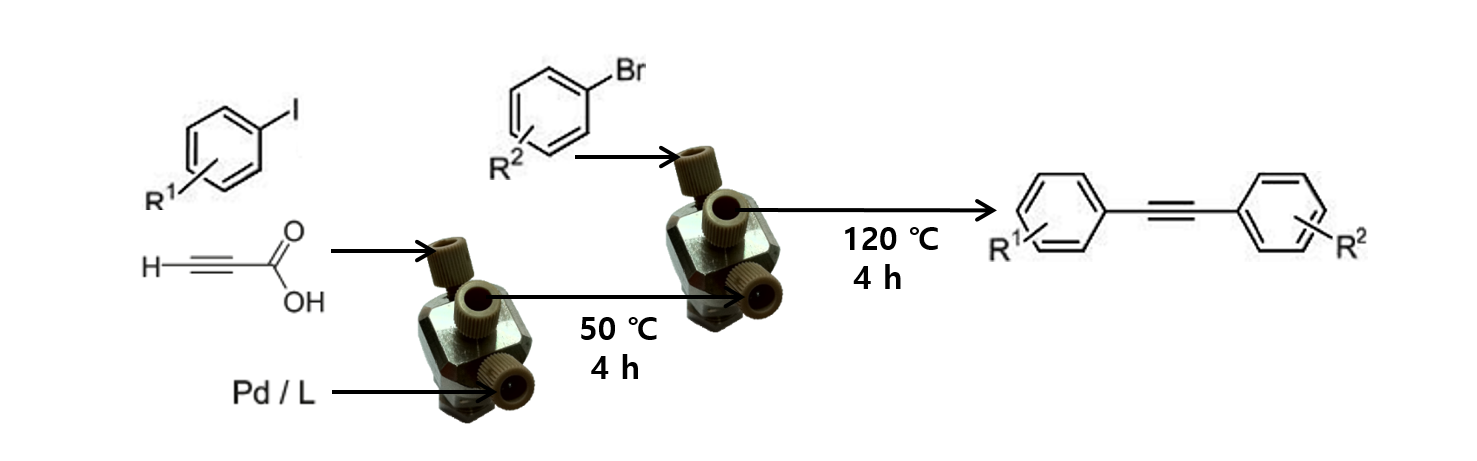

42. Kumar, M. R.; Park, A.; Park, N.; Lee, S. “Consecutive Condensation, C-N and N-N Bond Formations: A Copper-Catalyzed One-Pot Three-Component Synthesis of 2H-Indazole” Org. Lett. 2011, 13(13), 3542-3545. - Synfact
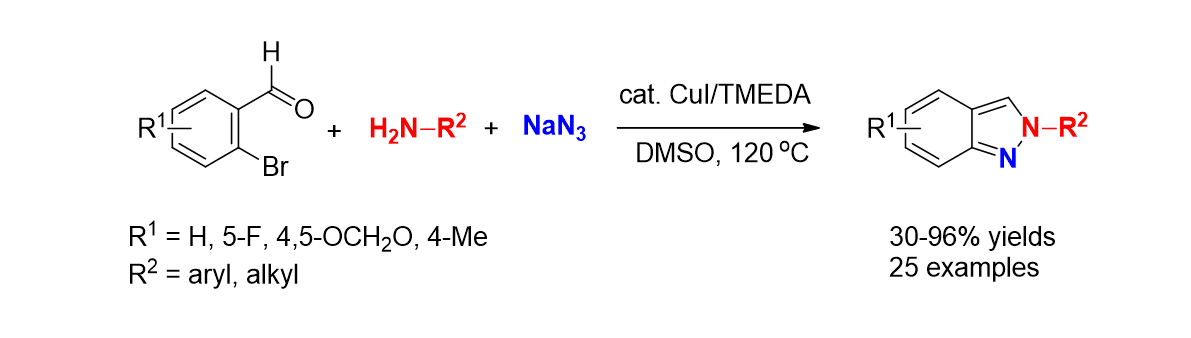

41. Park, N.; Park, K.; Jang, M.; Lee, S. "One-Pot synthesis of Symmetrical and Unsymmetrical Aryl Sulfides by Pd-Catalyzed Couplings of Aryl Halides and Thioacetates" J. Org. Chem. 2011, 76(11), 4371-4378. - Featured Article

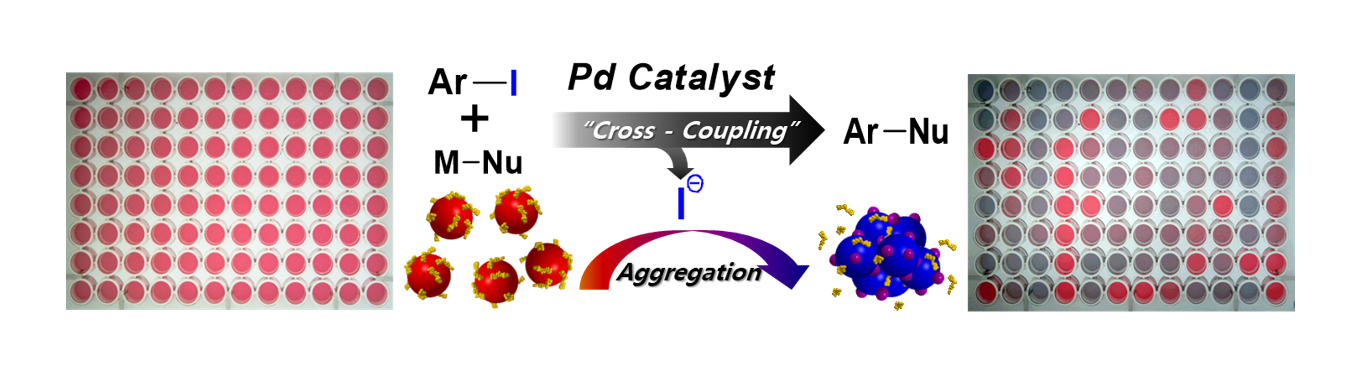
40. Jung, E.; Kim, S.; Kim, Y.; Seo, S. H.; Lee, S. S.; Han, M. S.; Lee, S. “A Colorimetric High-Throughput Screening Method for Palladium-Catalyzed Coupling Reactions of Aryl Iodides Using a Gold Nanoparticle-Based Iodide-Selective Probe” Angew. Chem. Int. Ed. 2011, 50(19), 4386-4389. - Nature Chemistry (Research Highlight)

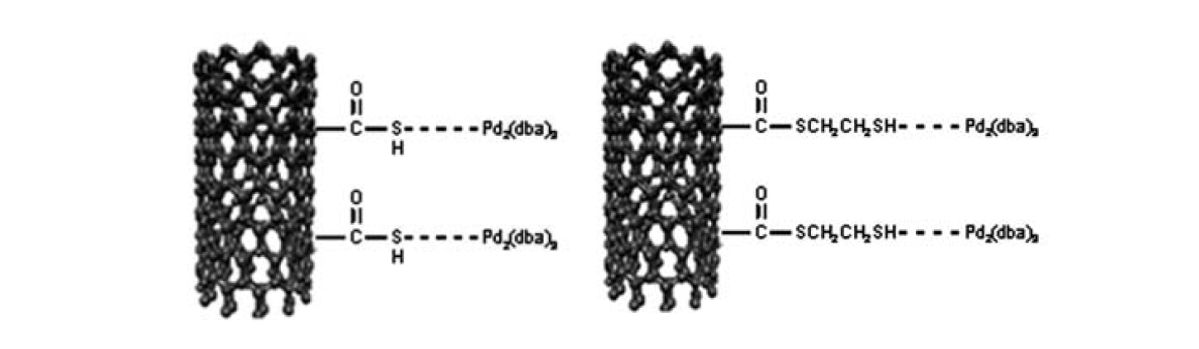
39. Kim, J. Y.; Park, K.; Bae, S. Y.; Kim, G. C.; Lee, S.; Choi, H. C. “Preparation, characterization and catalytic properties of Pd-decorated carbon nanotubes possessing different linkers” J. Mater. Chem. 2011, 21(16), 5999-6005.

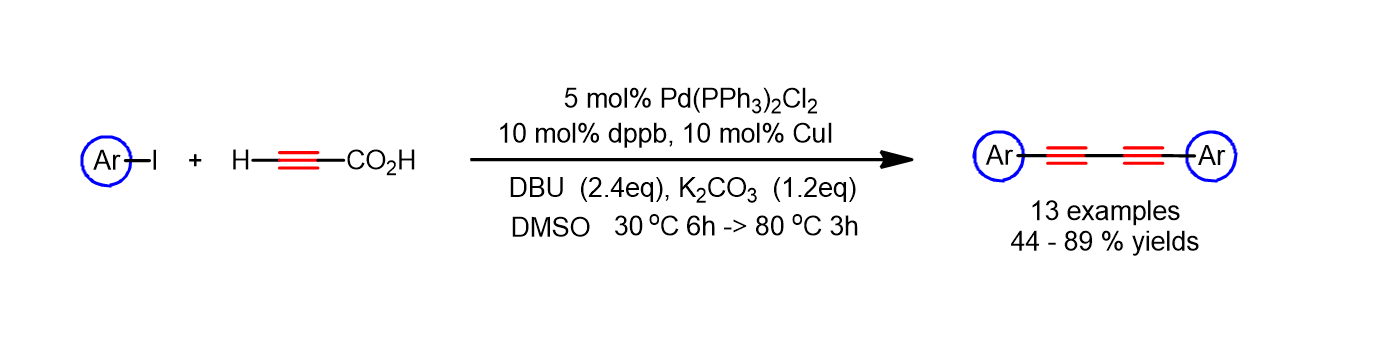
38. Kim, Y.; Park, A.; Park, K.; Lee, S. “One-Pot Synthesis of 1,4-Diarylsubstituted 1,3-Diynes from the Sequential Coupling Reactions of Aryl Iodides and Propiolic Acid” Teterahedron Lett. 2011, 52(15), 1766-1769.

37. Lu, J.; Kim, S.-G.; Lee, S.; Oh, I.-K. “Actuation of Electro-Active Artificial Muscle at Ultralow Frequency” Macromol. Chem. Phys. 2011, 212(6), 635-642.

36. Park, A.; Pak, K.; Kim, Y.; Lee, S. “Pd-Catalyzed Carbonylative Reactions of Aryl Iodides and Aryl Iodides and Alkynyl Carboxylic Acids via Decarboxylative Couplings” Org. Lett. 2011, 13(5), 944-947.
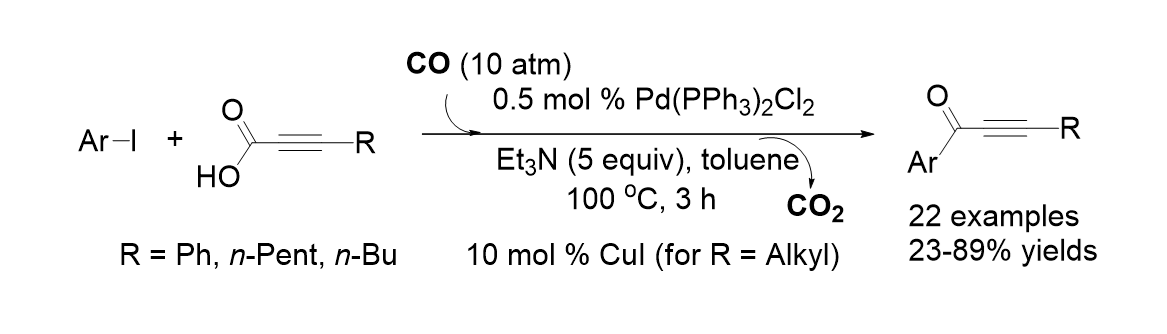

35. Kim, S. K.; Kim, Y. C.; Lee, S.; Kim, J. C.; Yun, M. Y.; Kim, I. S. “Insecticidal Activity of Phamnolipid Isolated from Pseudomonas sp. EP-3 against Green Peach Aphid (Myzus persicae)” J. Agric. Food Chem. 2011, 59(3), 934-938.

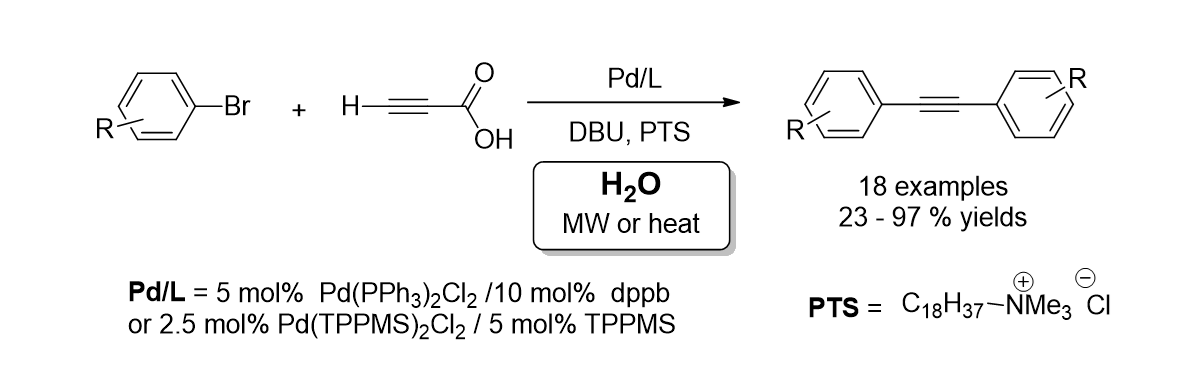
34. Park, K.; Bae, G.; Park, A.; Kim, Y.; Choe, J.; Song, K. H.; Lee, S. “Synthesis of Symmetrical Diarylalkyne from Palladium-Catalyzed Decarboxylative Couplings of Propiolic Acid and Aryl Bromides under Water” Teterahedron Lett. 2011, 52(5), 576-580.

[2010]

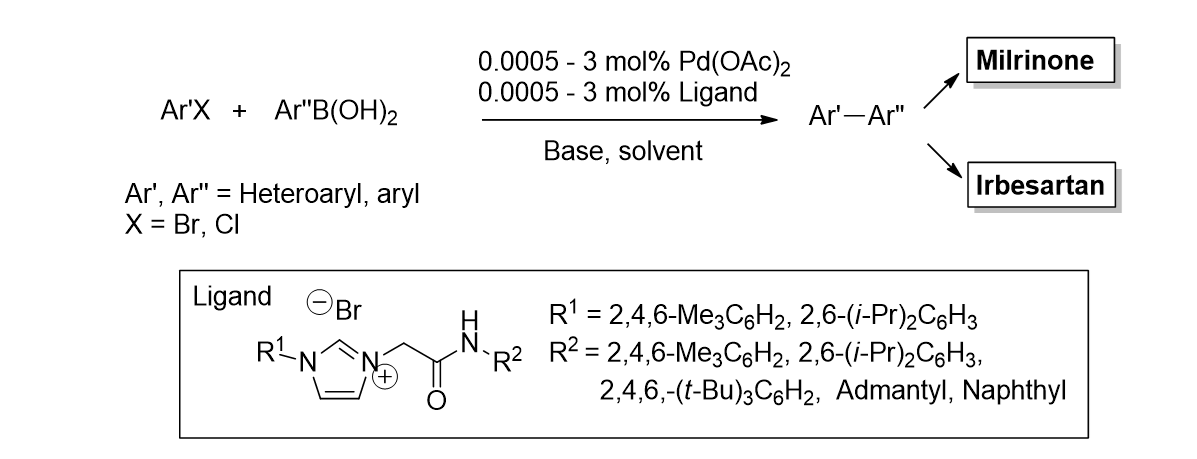
33. Kumar, M. R.; Park, K.; Lee, S. "Synthesis of amido-N-imidazolium salts and their applications as ligands in Suzuki-Miyaura reactions: Coupling of heteroaromatic halides and the synthesis of milrinone and irbesartan" Adv. Synth. Catal. 2010, 352, 3255-3266.

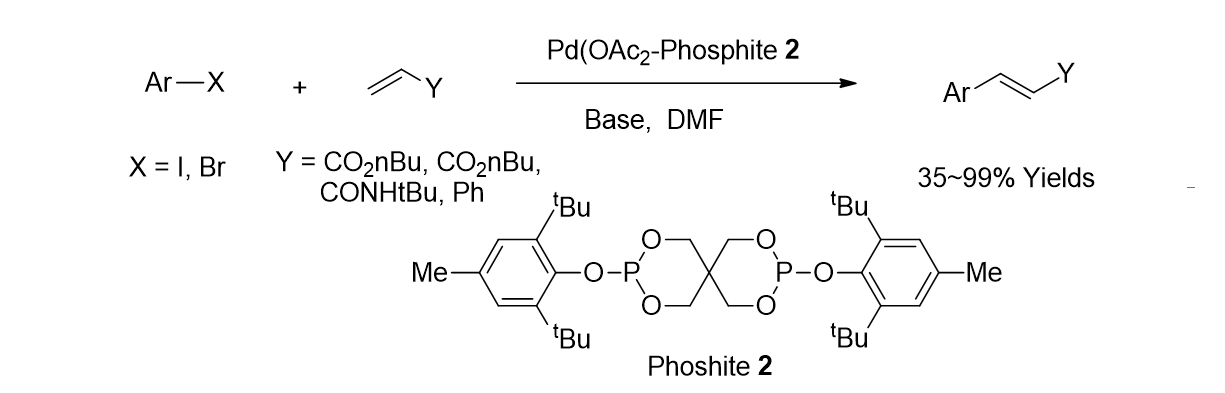
32. Jung, E.; Park, K.; Kim, J.; Jung, H.-T.; Oh, I.-K.; Lee, S. “Palladium-catalyzed Mizoroki-Heck coupling reactions using sterically bulky phosphite ligand” Inorg. Chem. Commun. 2010, 13, 1329-1331.

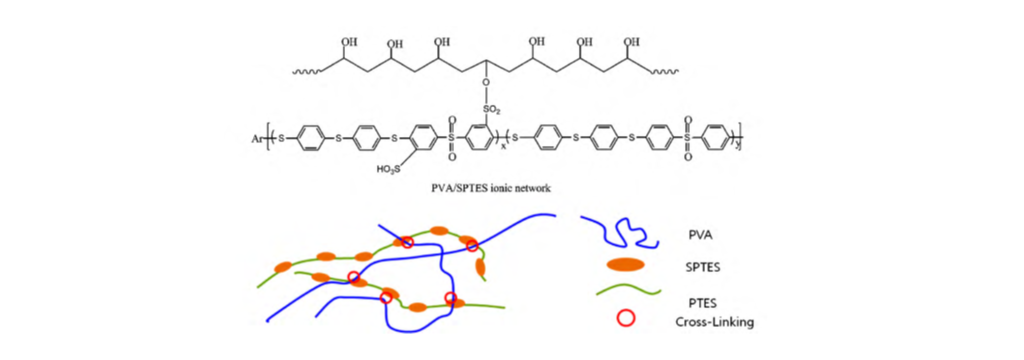
31. Wang, X.-L.; Oh, I.-K.; Lee, S. “Electroactive artificial muscle based on crosslinked PVA/SPTES” Sensor. Actuat. B.-Chem. 2010, 150(1), 57-64.

30. Park, K.; Bae, G.; Moon, J.; Choe, J.; Song, K. H.; Lee, S. “Synthesis of Symmetrical and Unsymmetrical Diarylalkynes from Propiolic Acid Using Palladium-Catalyzed Decarboxylative Coupling” J. Org. Chem. 2010, 75(18), 6244-6251.
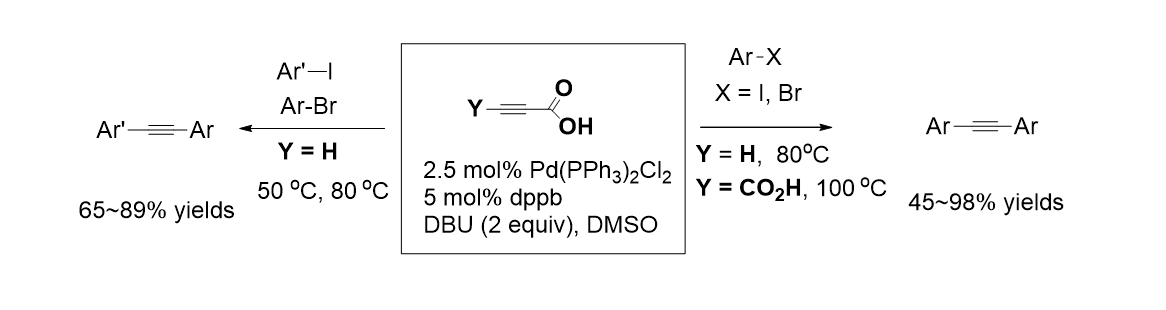

29. Song, E.; Park, J.; Oh, I.-K.; Jun, H. M.; Lee, S. “Ligand-free Palladium-Catalyzed Mizoroki-Heck-type Reaction of Arylboronic Acids and Alkenes Using Silver Cation” Bull. Korean Chem. Soc. 2010, 31(6), 1789-1792.
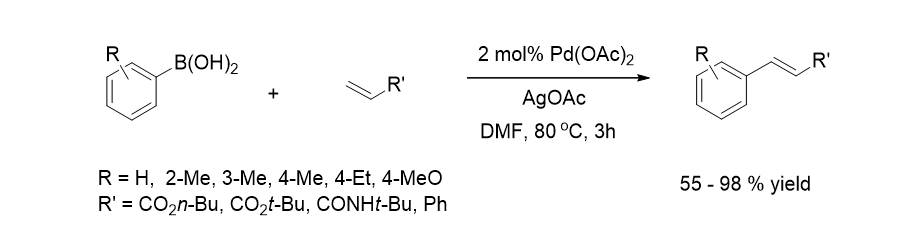

28. Jo, Y.; Kim, J. Y.; Oh, I.-K.; Choi, H. C.; Lee, S. “Ligand-Free Palladium Catalytic System Supported by CNT and its Application to the Mizoroki Heck Reactions” Bull. Korean Chem. Soc. 2010, 31(6), 1735-1738.
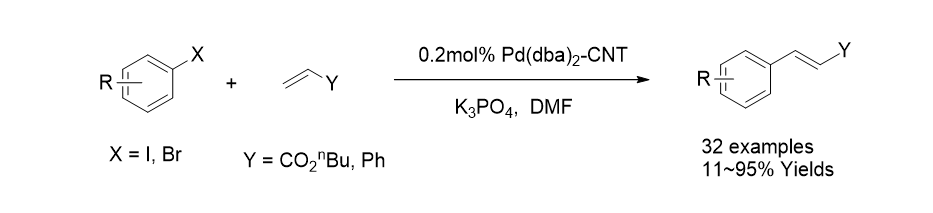

27. Kim, J. Y.; Jo, Y.; Kook, S.-K.; Lee, S.; Choi, H. C. “Synthesis of carbon nanotube supported Pd catalysts and evaluation of their catalytic properties for C-C bond forming reactions” J. Mol. Catal. A.: Chem. 2010, 323, 29-32.

26. Yang, G. R.; Bae, G.; Choe, J.; Lee, S.; Song, K. H. “Silica-Supported Palladium-Catalyzed Hiyama Cross-Coupling Reactions Using Continuous Flow System” Bull. Korean Chem. Soc. 2010, 31(1), 250-252.
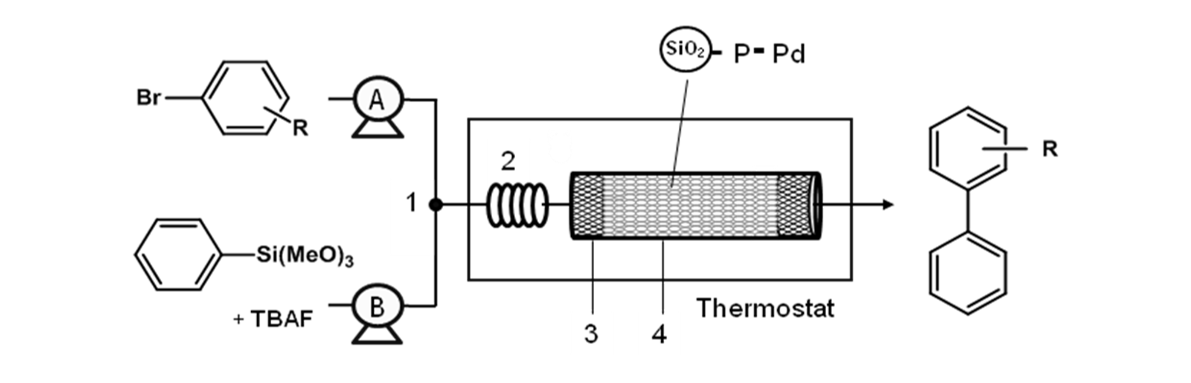

[2009]


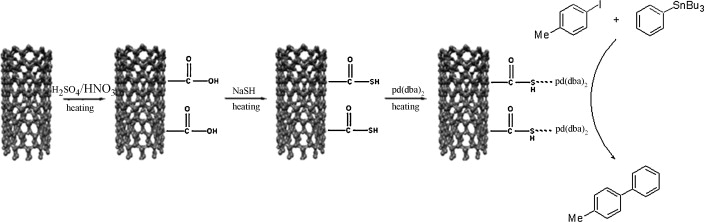
25. Kim, J. Y.; Jo, Y.; Lee, S.; Choi, H. C. "Synthesis of Pd-CNT nanocomposites and investigation of their catalytic behavior in the hydrodehalogenation of aryl halides" Tetrahedron Lett. 2009, 50(46), 6290-6292.

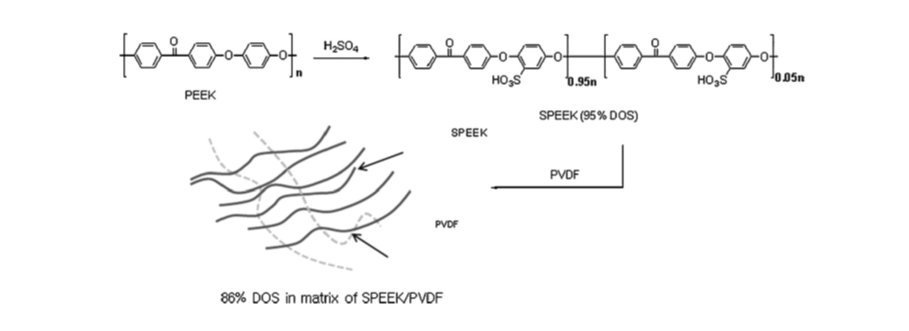
24. Jeon, J.-H.; Kang, S.-P.; Lee, S.; Oh, I.-K. "Novel biomimetic actuator based on SPEEK and PVDF" Sensor Actuat. B.-Chem. 2009, 143, 357-364.

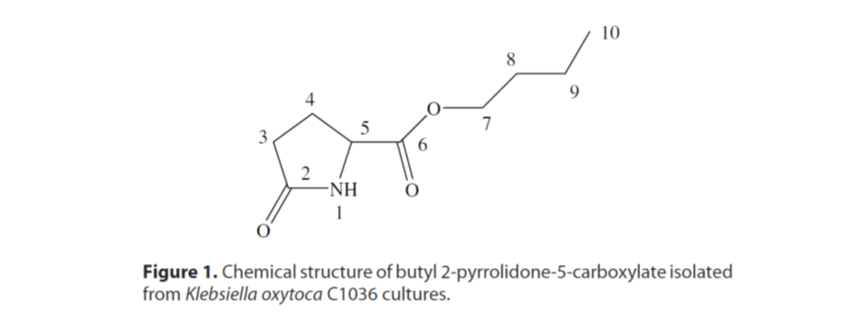
23. Park, M.; Kim, Y. C.; Lee, S.; Kim, I. S. "Identificatio of an ISR-related metabolite produced by rhizobacterium Klebsiella oxytoca C1036 active against soft-rot disease pathogen in tobacco" Pest. Manag. Sci. 2009, 65(10), 1114-1117.

22. Song, E.; Jo, Y.; Bae, G.; Oh, I.-K.; Jung, H. M.; Lee, S. " Synthesis of Phosphionodiselenoic Acid Ester Derivatives and their Application in the Controlled Radical Polymerization of Styrene" Bull. Korean Chem. Soc. 2009, 30(9), 2129-2131.
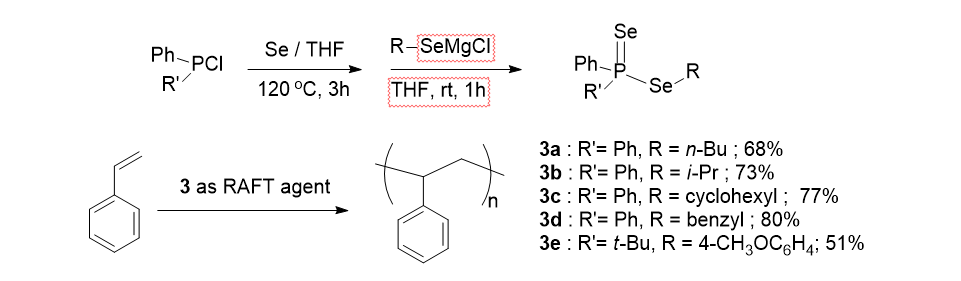

21. Jo, Y.; Ju, J.; Choe, J.; Song, K. H.; Lee, S. "The Scope and Limitation of Nickel-Catalyzed Aminocarbonylation of Aryl Bromides from Formamide Derivatives" J. Org. Chem. 2009, 74(16), 6358-6361.
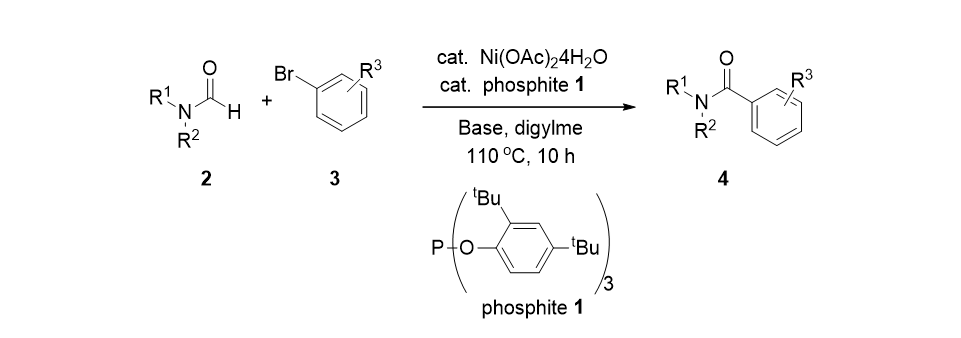

20. Jang, M.; Jo, Y.; Oh, I.-K.; Jung, H. M.; Lee, S. "Suzuki-Miyaura Coupling Reactions Using Phosphite Ligands" Synthesis 2009, (12), 2073-2075.
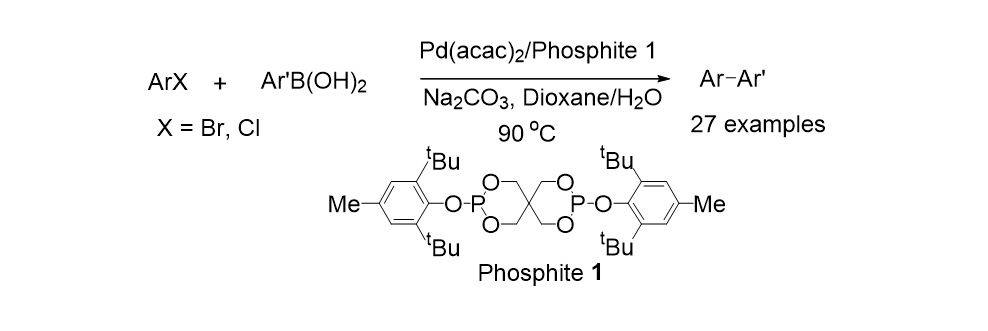

19. Moon, J.; Jang, M.; Lee, S. " Palladium Catalyzed Decarboxylative Coupling or Alkynyl Carboxylic Acids and Aryl Halides" J. Org. Chem. 2009, 74(3), 1403-1406.
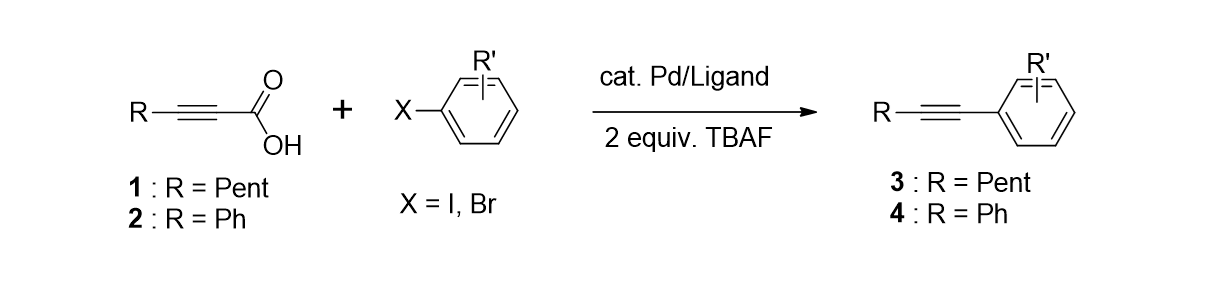

18. Moon, J.; Lee, S. "Palladium Catalyzed Dehalogenation of Aryl Chlorides and Bromides Uisng Phosphite Ligands" J. Organomet. Chem. 2009, 694(3), 473-477.
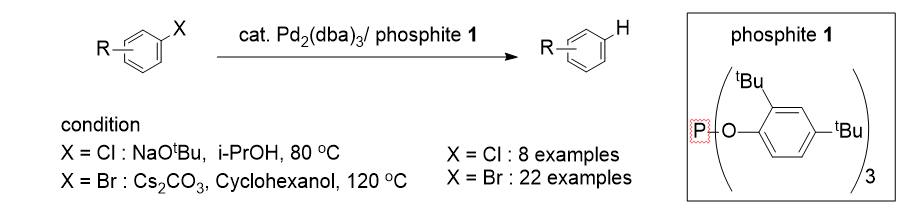

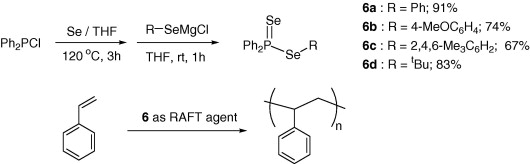
[2008]


17. Moon, J.; Nam, H.; Kim,S.; Ryu, J.; Han, C.; Lee, C.; Lee, S. “Synthesis of phosphinodiselenoic acid esters and their application as RAFT agents in styrene polymerization” Tetrahedron Lett. 2008, 49(35), 5137-5140.

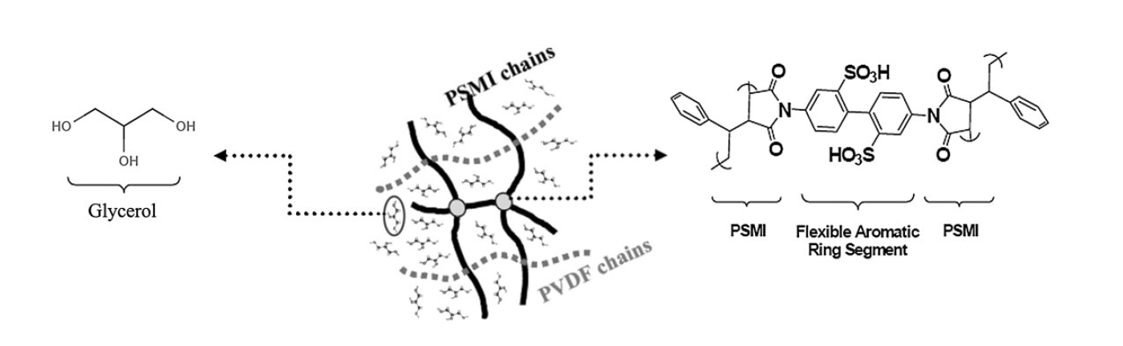
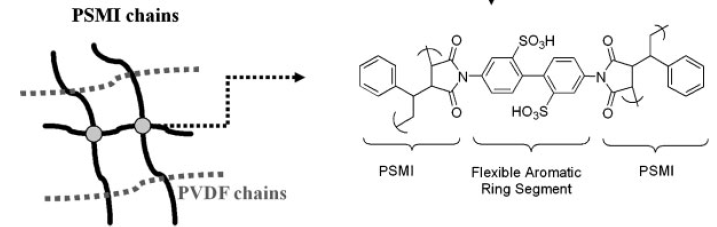
16. Lu, J.; Kim, S.-G. Lee, S.; Oh, I.-K. "Fabrication and actuation of electro-active polymer actuator based on PSMI-incorporated PVDF" Smart. Mater. Struct. 2008, 17(4), 045002/1-045002/10.


15. Lu, J.; Kim, S.-G.; Lee, S.; Oh, I.-K. “A biomimetic actuator based on an ionic networking membrane of poly(styrene-alt-maleimide)-incorporated poly(vinlylidene) fluroide” Adv. Funct. Mater. 2008, 18(8), 1290-1298.

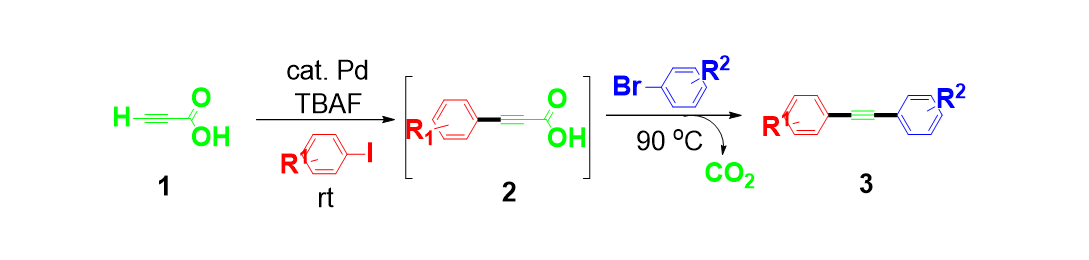
14. Moon, J.; Jeong, M.;Nam, H.; Ju, J.; Moon, J. H.; Jung, H. M.; Lee, S. “One-Pot Synthesis of Diarylalkynes Using Palladium-Catalyzed Sonogashira Reaction and Decarboxylative Coupling of sp and sp2 Carbon” Org. Lett. 2008, 10(5), 945-948.- Synfact

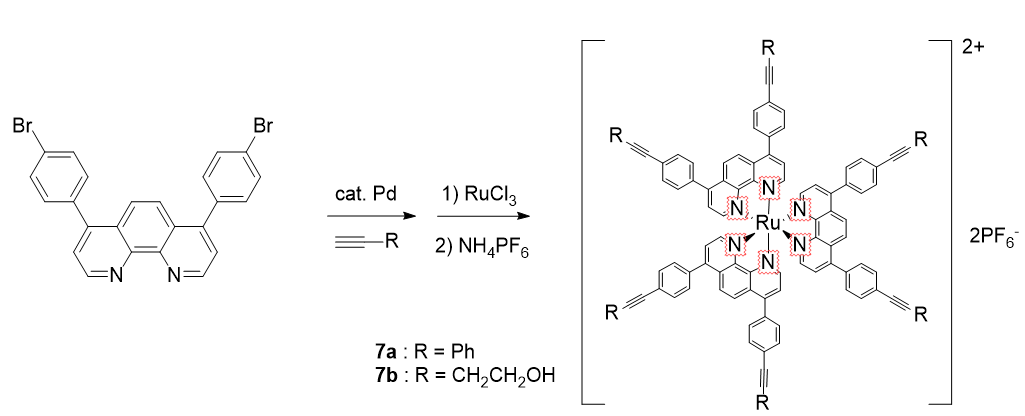
13. Jeong, M.;Nam, H.; Sohn, O.-J.; Rhee, J. I.; Kim, H. J.; Cho, C.-W.; Lee, S. “Synthesis of phenanthroline derivatives by Sonogashira reaction and the use of their ruthenium complexes as optical sensors” Inorg. Chem. Comm. 2008, 11(1), 97-100.

[2007]


12. Moon, J.;Nam, H.; Ju, J.; Jeong, M.; Lee, S. “Homocoupling of Aryl Halides Using Catalytic System of Palladium and Phosphite” Chem. Lett. 2007, 36 (12), 1432-1433.

11. Shin, J.; Jensen, S. M.; Ju, J.; Lee, S.; Xue, Z.; Noh, S. K.; Bae, C. “Controlled Functionalization of Crystalline Polystyrenes via Activation of Aromatic C-H Bonds” Macromolecules, 2007, 40(24), 8600-8608.

10. Wang, X.-L.; Oh, I.-K.; Lu, J.; Ju, J.; Lee, S. “Biomimetic electro-active polymer based on sulfonated poly(styrene-b-ethylene-co-butylene-b-styrene)” Mater. Lett. 2007, 61(29), 5117-5120.

9. Ju, J.; Jeong, M.; Moon, J.; Jung, H. M.; Lee, S. “Aminocarbonylation of Aryl Halides Using a Nickel Phosphite Catalytic System” Org. Lett. 2007, 9(22), 4615-4618.

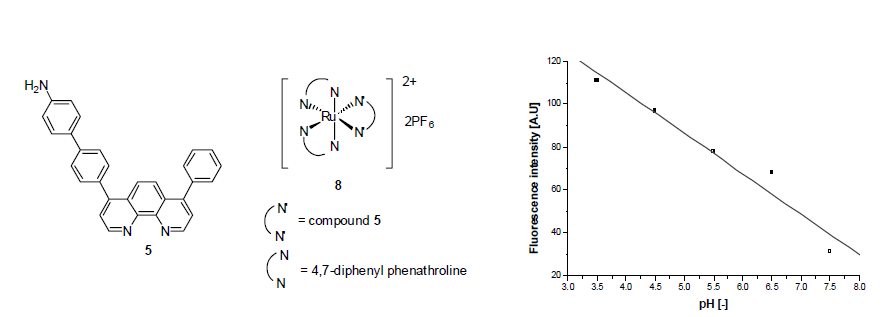
8. Jeong, Y. C.; Sohn, O,-J.; Rhee, J. I.; Lee, S.; Kim, H. J. "Optical Sensing material for dissolved oxygen: covalent immobilization of tris(4,7-diphenyl-1,10-phenanthroline) ruthenium(II) complex in sol-gels" Bull. Korean Chem. Soc. 2007, 28(5), 883-886.

7. Jeon, J.-H.; Oh, I.-K.; Han, J.-H.; Lee, S. “Development of bio-mimetic patterned IPMC actuators with multiple electrodes” Key. Eng. Mat. 2007, 334-335, 1005-1008.


6. Jo, C.; Jo. Y. J.; Park, H.-R.; Lee, J.-B.; Kwon, Y.-H.; Lee, S.; Chun, K. H.; Oh, J. “Synthesis and evaluation of unsatuarated alkyl esters of 5-aminolevulinic acid as precursors to protoporphyrin IX.” Bull. Korean Chem. Soc. 2007, 28(1), 129-132.

5. Nam, H.; Jeong, M.; Sohn O.; Rhee, J.; Oh, J.; Kim, Y.; Lee, S. "Synthesis of phenanthroline derivative by Suzuki coupling reaction and the use of its ruthenium complex as an optical pH sensor" Inorg. Chem. Commun. 2007, 10, 195- 198.



4. Ju, J.; Nam, H.; Jung, H. M.; Lee, S. "Palladium-catalyzed cross-coupling of trimethoxysilylbenzene with aryl bromides and chlorides usingphosphite ligands" Tetrahedron Lett. 2006, 47, 8673-8676.
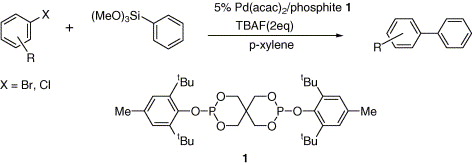

3. Park, M. R.; Lee, S.; Han, T.-H.; Oh, B.-T.; Shim, J. H.; Kim, I. S. "A New Intermediate in the Degradation of Carbofuran by Sphingomonas sp. Strain SB5" J. Microbiol. Biotechnol. 2006, 16, 1306 - 1310
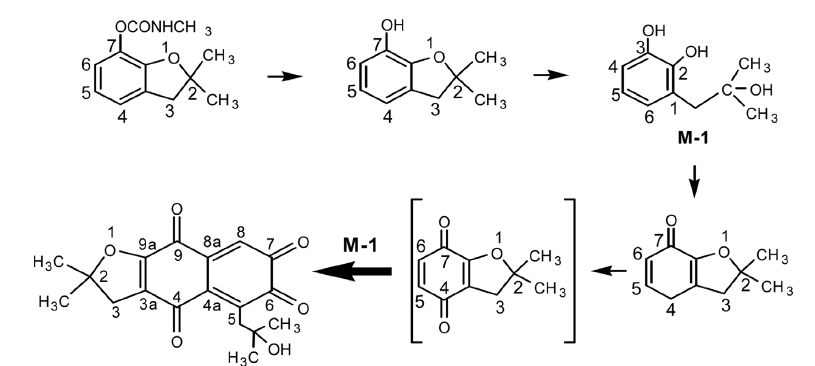

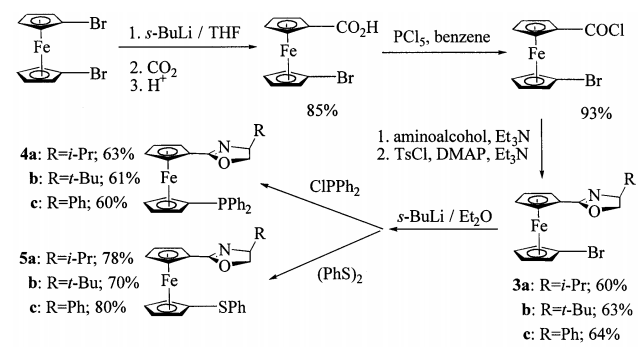
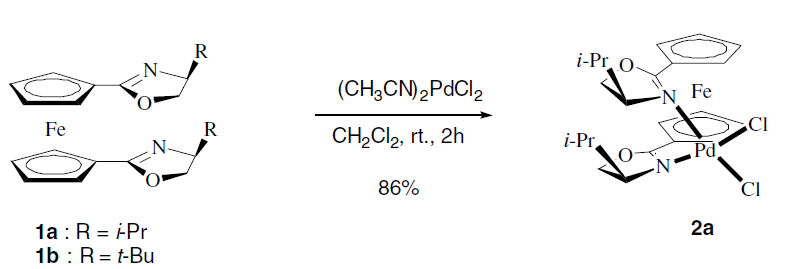
2. Lee, S. "1,1-Bis(oxazolinyl)ferrocene-based palladium catalysts: Synthesis, X-ray structures and applications in Suzuki and Heck coupling reactions" J. Organomet Chem. 2006, 691, 1347-1355.

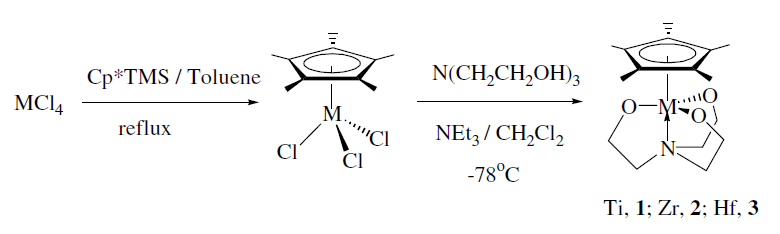
1. Lee, K.-S. Kim, Y.; Ihm, S.-K.; Do, Y.; Lee, S. "New group 4 half sandwich complexes containing triethanolamine ligand for polyethylene" J. Organomet. Chem. 2006, 691, 1121-1125.

At Yale


5. Joergensen M.; Lee S.; Liu, X.; Wolkowski, J. P.; Hartwig, J. F. "Efficient Synthesis of α-Aryl Esters by Room-Temperature Palladium-Catalyzed Coupling of Aryl Halides with Ester Enolates" J. Am. Chem. Soc. 2002, 124, 12557-12565.
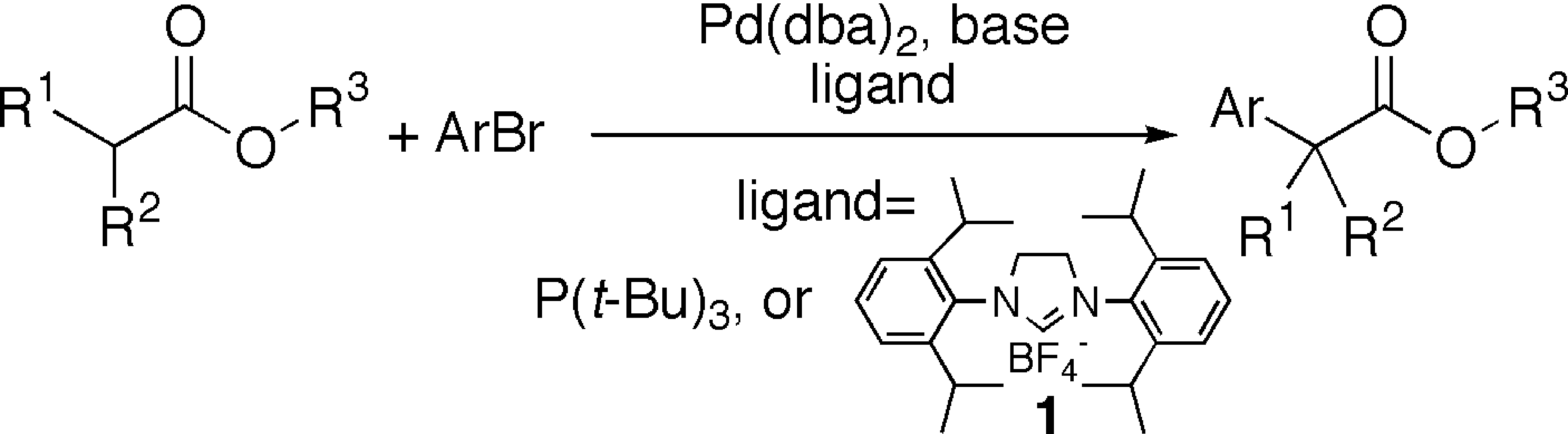

4. Lee, S.; Beare, N.A.; Hartwig, J.F. "Palladium-Catalyzed alpha-Arylation of Esters and Protected Amino Acids" J. Am. Chem. Soc. 2001, 123, 8410-8411.
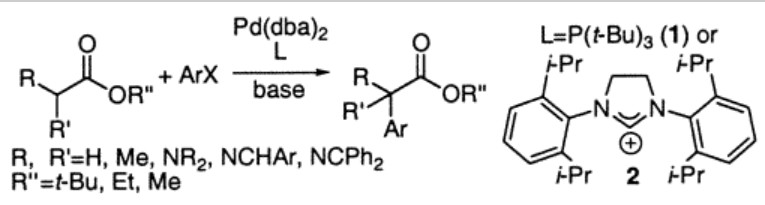

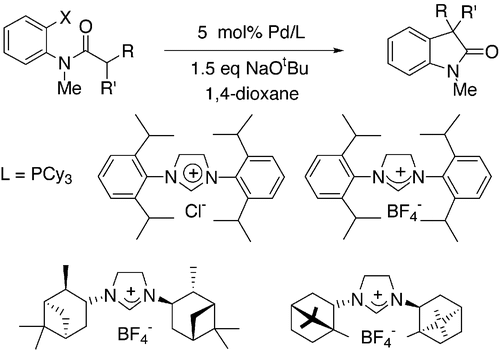
3. Lee, S.; Hartwig, J.F. "Improved Catalysts for the Palladium-Catalyzed Synthesis of Oxindoles by Amide-Arylation. Rate Acceleration, Use of Aryl Chloride Substrates and a New Carbene Ligand for Asymmetric Transformations" J.Org. Chem. 2001, 66, 3402-3415.

2. Lee, S.; Joergensen, M.; Hartwig, J. F. "Palladium-Catalyzed Synthesis of Arylamines from Aryl Halides and Lithium Bis (trimethylsilyl)amide as an Ammonia Equivalent" Org. Lett. 2001, 3, 2729-2732.


1. Stauffer, S.R.; Lee, S.; Stambuli, J.P; Hauck, S.I.; Hartwig, J. F. "High turnover number and rapid, room-temperature amination of choroarenes using saturated carbene ligands" Org. Lett. 2000, 2, 1423-1426.
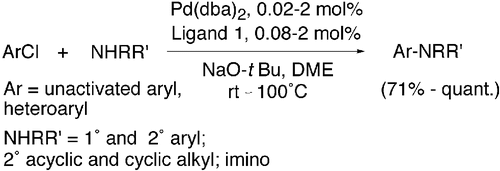

At POSTECH


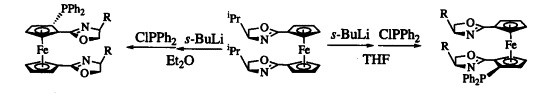
7. Lee, S.; Koh, J. H.; Park, J. "ortho-Silylation of 2, 2'-Bis(oxazolinyl)-1, 1'-bis(diphenylphosphino)ferrocenes and Remarkable Effect of the Silyl Groups on the Enantioselectivity in Pd-Catalyzed Asymmetric Allylic Alkylation," J. Organomet. Chem. 2001, 637-639, 99-106.
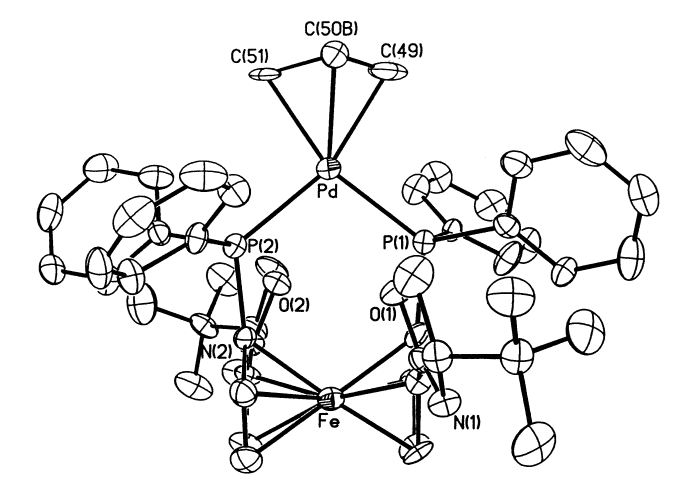

6. Park, J.; Quan, Z.; Lee, S.; Ahn, K. H.; Cho, C.-H. "Synthesis of chiral 1'-substituted oxazolinylferrocenes as chiral ligands for Pd-catalyzed allylic substitution reactions" J. Organomet. Chem. 1999, 584, 140-146.
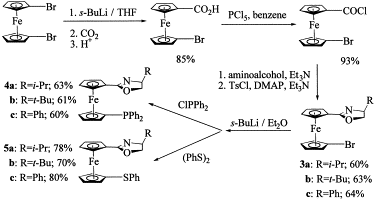

5. Ahn, K. H.; Cho, C.-W.; Park, J.; Lee, S. "Palladium-catalyzed asymmetric allylic alkylations using diphenylphosphino (oxazolinyl) ferrocene ligands: effects of planar chirality on the reactivity and selectivity" Bull. Kor. Chem. Soc. 1997, 18, 789-791.
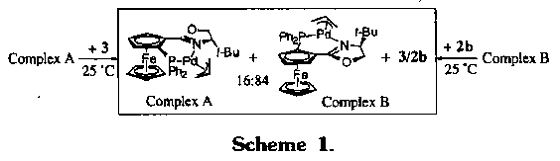

4. Ahn, K. H.; Cho, C.-W.; Park, J.; Lee, S. "Pd-catalyzed asymmetric allylic alkylations using various diphenylphosphino(oxazolinyl)ferrocene ligands." Tetrahedron Asymmetry, 1997, 8, 1179-1185.
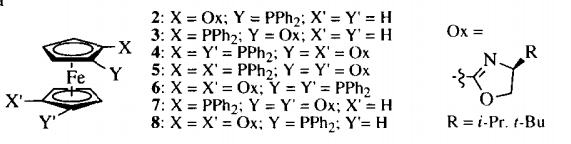

3. Ahn, K. H.; Cho, C.-W.; Baek, H.-H. Park, J.; Lee, S. "An Efficient Diastereoselective Synthesis of Chiral (Oxazolinyl)ferrocene Compounds." J. Org. Chem. 1996, 61, 4937-4943.
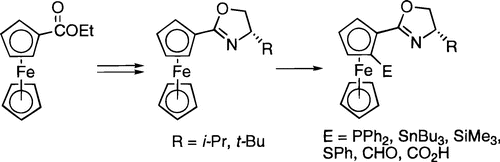

2. Park, J.; Lee, S.; Ahn, K. H.; Cho, C.-W. "Effects of solvent and lithiating agent on stereoselectivity in lithiation of chiral 1, 1'-bis(oxazolinyl)ferrocenes." Tetrahedron Lett. 1996, 37, 6137-6140.